Stiff and Tough Ion Gels for Electrolyte Membranes of Flexible Batteries
Mayumi Group
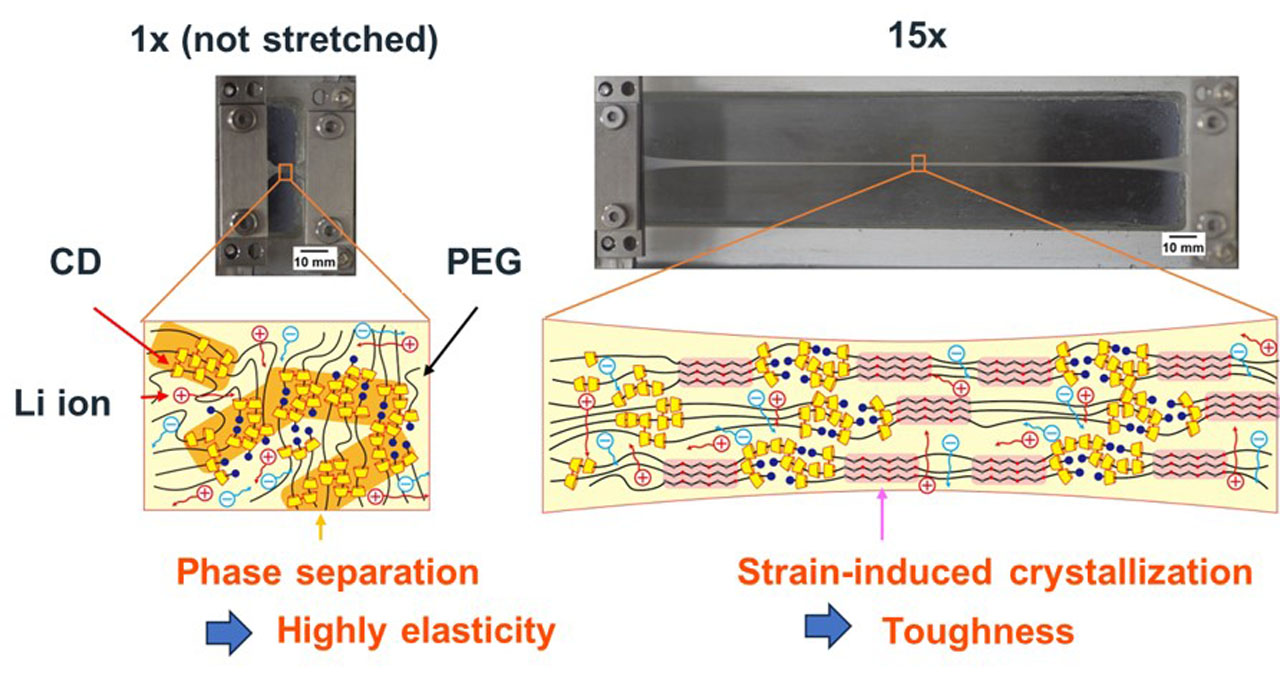
Gel electrolytes consist of polymer network and ion-conductive liquids as solvent. Due to their flexibility, they have been applied for electrolyte membranes of flexible Li ion batteries that can be attached to our skin or clothing. Electrolyte membranes require a high elastic modulus to prevent Li metal crystals from growing at the electrode surface during charging and discharging, causing a short circuit in the batteries. Previous research has reported that the formation of lithium metal crystal is suppressed for electrolyte membranes with an elastic modulus of 10 MPa or more. In addition, to prevent crack growth during repeated deformation of flexible batteries, gel electrolyte membranes need to exhibit high fracture toughness. For conventional gel electrolytes, polymer crystallization was used to increase stiffness. However, hard gel electrolytes tend to become brittle, and it has been difficult to achieve both stiffness and toughness. Although various high-strength gel electrolytes have been developed, no gel electrolytes achieved high elastic modulus (over 10 MPa) and fracture toughness. In our laboratory, by combining phase separation and strain-induced crystallization, we have succeeded in developing a gel electrolyte that exhibit a high elastic modulus of over 10 MPa and high toughness of about 100 MJ/m3 (Fig. 1) [1]. When our gel electrolyte is deformed, stretched polymer chains form crystals, which improvs the mechanical toughness [2]. To homogenize chain deformation in the gel electrolytes, we used slide-ring (SR) network in which polymer chains are connected by ring molecules. The ring molecules of the SR network are aggregated to form a hard continuous phase, which resulted in a high elastic modulus (70 MPa). Our gel electrolytes are sufficiently stiff to prevent short circuits during charging and discharging of the batteries, and are also tough enough to withstand repeated deformation, which leads to improved durability of flexible batteries (Fig. 2).
References
- [1] K. Hashimoto, T. Shiwaku, H. Aoki, H. Yokoyama, K. Mayumi, and K. Ito, Sci. Adv. 9, eadi8505 (2023).
- [2] C. Liu, N. Morimoto, L. Jiang, S. Kawahara, T. Noritomi, H. Yokoyama, K. Mayumi, and K. Ito, Science 372, 1078 (2021).