Structural Characterization of Module-Assembled Amphiphilic Conetwork Gels
M. Shibayama, S. Kondo, and E. P. Gilbert
Amphiphilic conetworks are composed of hydrophilic and hydrophobic polymers. Their amphiphilicity is promising for many applications because amphiphilic conetworks can absorb both polar and nonpolar solutes. This is useful for drug delivery, drug release systems, antifouling coatings, gas and biosensors, chiral separation membranes, activating carriers for biocatalysts, and soft contact lenses. We developed inhomogeneity-free amphiphilic conetworks consisting of poly(ethylene glycol)−poly(dimethylsiloxane) (PEG−PDMS) and carried out structural analysis by the complementary use of small-angle X-ray (SAXS) and neutron scattering (SANS). Figure 1 shows the preparation scheme of PEG−PDMS gels. The main components were two types of tetra-arm PEGs; −COO-NHS terminated PEG (NHS: N-hydroxysuccinimide) and −NH2 terminated PEG. In addition, the weight fraction of PDMS in the conetworks, r, was tuned by using −NH2 terminated linear-PEG. By using equimolar prepolymers carrying −COO-NHS and −NH2, a precise tuning of PDMS content rate was achieved. Because of the hydrophobicity of PDMS units, the PEG−PDMS gels exhibit a microphase-separated structure in water. Figure 2 shows the SANS profiles of swollen PEG−PDMS gels with various solvents. The SANS profiles exhibited marked changes depending on the solvent; from a monotonous decrease without peak for toluene to multiple peaks for water.
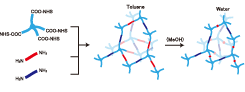
Fig. 1. Schematics of sample preparation for PEG−PDMS gels with r = 0.5. Light blue, dark blue, and red segments represent tetra-arm-PEG, linear-PEG, and linear-PDMS units, respectively. In toluene, the gels are in swollen state, while they undergo microphase separation in water due to shrinking of the PDMS chains.
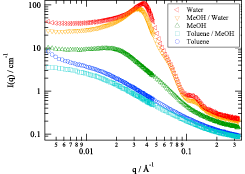
Fig. 2. SANS profiles of PEG−PDMS gels (r = 1) with different solvents. The ratios of toluene/MeOH and MeOH/water are both 1/1 (volume fraction). By decreasing the solubility of PDMS, the gels undergo microphase separation, giving rise to distinct peaks in SANS.
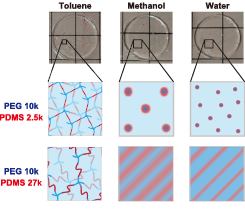
Fig. 3. Proposed phase separation mechanism during solvent substitution from toluene, methanol, to water. The morphology is dependent on the ratio of PEG and PDMS as well as their molecular weights.
Depending on the volume fraction of PDMS, the microphase-separated structure varies from core−shell to lamellar. The obtained SAXS and SANS profiles are reproduced well using a core−shell model together with a Percus−Yevick structure factor when the volume fraction of PDMS is small. The domain size is much larger than the size of individual PEG and PDMS unit, and this is explained using the theory of block copolymers. Reflecting the homogeneous dispersion conditions in the as-prepared state, scattering peaks are observed even at a very low PDMS volume fraction (0.2 %). When the volume fraction of PDMS is large, the microphase-separated structure is lamellar and is demonstrated to be kinetically controlled by nonequilibrium and topological effects. The microphase separation in the PEG−PDMS gels is illustrated schematically in Figure 3. It is clarified that the microphase-separated structure is tunable by changing the molecular weight of PEG and PDMS and their proportion. This may open the door for the precise design of the mesoscopic structure of amphiphilic gels.
References
- [1] T. Hiroi, S. Kondo, T. Sakai, E. P. Gilbert, Y. S. Han, T. H. Kim, and M. Shibayama, Macromolecules 49, 4940 (2016).
