Kinetic Aspect on Gelation Mechanism of Tetra-PEG Hydrogel
Shibayama Group
Gels are defined as three-dimensional polymer networks swollen in solvent. Because of their high retention capacity of solvents, gels are utilized as super absorbent, contact lenses, drug reservoirs, etc. However, industrial applications of gels are still limited because of their low mechanical strength, which results from inhomogeneity created during the cross-linking process. One of the most popular approaches for removing inhomogeneity is an “end-crosslinking” method, which forms a polymer network from AB-type polycondensation of telechelic prepolymers and multifunctional crosslinkers. Because of simplicity of its network structure, mechanical, structural and simulational studies were conducted on this type of polymer network. However, from these studies, it is found that this network still contains inhomogeneities such as spatial concentration fluctuation, connectivity defects, and entanglements. We developed a novel class of hydrogels by “cross-end-coupling” of two types of tetra-arm poly(ethylene glycol) (PEG) units that have mutually reactive amine (Tetra-PEG-NH2) and activated ester (Tetra-PEG-NHS) terminal groups, respectively [1]. A schematic illustration of the tetra-PEG gel is shown in Fig. 1, where the red and blue lines represent the component chains.
Fig. 1. Schematic illustration showing tetra-arm poly(ethylene glycol) (Tetra-PEG) gel. The red and blue lines represent Tetra-PEG-NH2 and Tetra-PEG-NHS chains, respectively. Balls denotes the space occupied by the polymer chains.
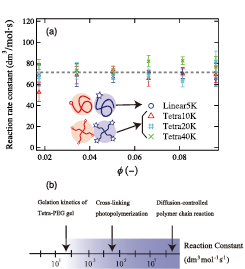
Fig. 2. (a) The reaction rate constant, kgel, plotted against polymer concentration φ for 5K LinearPEG, 10K Tetra-PEG, 20K Tetra-PEG, and 40K Tetra-PEG. (b) Comparison of reaction rate constants of Tetra-PEG gel, cross-linking photopolymerization, and diffusion-controlled polymer chain reaction.
In order to elucidate the high reactivity and high regularity of the Tetra-PEG gels, we carried out a kinetic study on the gelation reaction of the two tetra-arm poly(ethylene glycol) (Tetra-PEG) prepolymers by ATR-IR and UV spectroscopies [2]. The reaction rate constant for the gelation of Tetra-PEG was determined in aqueous solutions with varying both prepolymer volume fraction, φand molecular weight, Mw, of the prepolymers. The gelation rate constant, kgel was successfully estimated for all the systems. Figure 2(a) clearly shows that the kgel values obtained here are almost constant within experimental error regardless of polymer volume fraction, φ, and prepolymer molecular weight, Mw, and their kgel values for Tetra-PEG gel are essentially consistent with those for linearPEG system (Linear5k). The value of kgel for Tetra-PEG gel is obtained to be around 70 dm3 mol-1 s-1, which is much smaller than the reaction rate of typical diffusion-controlled reaction (e.g., 108 ~ 109 dm3 mol-1 s-1) and cross-linking photopolymerization (104 ~ 105 dm3 mol-1 s-1). That is, the reaction rate of the amide bond formation is much slower than the collision rate of the terminal NHS and amine groups. From these results, we concluded that the gelation reaction of Tetra-PEG gel is not diffusion-limited but reaction-limited process, i.e. the diffusion motion is much faster than the reaction rate (Fig. 2(b)). It is thus expected that Tetra-PEG prepolymer chains can diffuse in the solution during gelation process, leading to homogeneity and high-strength of Tetra-PEG gel. These discussions imply that, in order to achieve high-efficient and homogeneous gel, it is necessary to choose reaction groups so as to undergo reaction-limited reaction.
References
- [1] T. Sakai et al., Macromolecules 41, 5379 (2008).
- [2] K. Nishi et al., Macromolecules 47, 3274 (2014).
