Selective Probing of the OH or OD Stretch Vibrations in Liquid Water Using Resonant Inelastic Soft X-ray Scattering
Y. Harada, T. Tokushima, and S. Shin
The unique property of water, such as high boiling and melting point compared to molecules like non-metal hydride, or less density in solid form than in liquid form, are explained by an attractive force between water molecules, called 'hydrogen bond'. There are many proposed local structural models that describe the water network. Among them, a continuum model, where hydrogen bonds in water are distorted, broken and reformed continuously, but water itself is composed of a single component, or a mixture (micro-heterogeneity) model, where the network is considered as a mixture of various hydrogen bond configurations, are well known. However, the model that describes better the hydrogen bond property of liquid water, is still under debate.
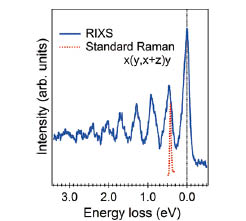
Fig. 1. The water vibronic structure observed in the RIXS spectrum. Dotted red curve is a standard Raman spectrum of liquid water in the same experimental configuration x(y, x+z)y [2] as the O 1s RIXS.
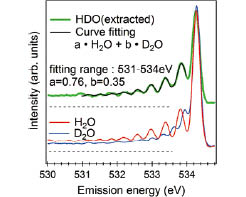
Fig. 2. Isotope effect on the multiple vibrational excitations of water. For each spectrum the background level is indicated by a dashed line. The extracted HDO spectrum is fitted with a linear combination of the H2O and D2O spectra in the range from 531–534 eV.
In this work, we have succeeded in revealing validity of the mixture (micro-heterogeneity) model by selective observation of hydrogen-bond-broken water molecules and in detecting with high sensitivity the difference in the degree of hydrogen bond strength between light (normal) water and heavy water (in which both hydrogen atoms have been replaced with deuterium) using soft X-ray resonant inelastic scattering at BL07LSU and BL17SU of SPring-8[1]. High-resolution O 1s resonant inelastic X-ray scattering spectra of liquid H2O/D2O/HDO, obtained by excitation near the pre-edge resonance (Fig. 1) show, in the elastic line region, well-separated multiple vibrational structures corresponding to the internal OH stretch vibration in the ground state of water. The energy of the first-order vibrational excitation is strongly blue-shifted with respect to the main band in the Infrared/Raman spectra of water [2], indicating that water molecules with a highly weakened or broken donating hydrogen bond are correlated with the pre-edge structure in the X-ray absorption spectrum. As shown in Fig. 2, the vibrational profile of pre-edge excited HDO water is well fitted with 50±20% greater OH-stretch contribution compared to OD, which strongly supports a preference for OH being the weakened or broken H-bond in agreement with the well-known picture that D2O makes stronger H-bonds than H2O [3,4]. Accompanying path-integral molecular dynamics simulations show that this is particularly the case for strongly asymmetrically H-bonded molecules, i.e. those that are selected by pre-edge excitation. These results are expected to lead to the clarification of the role of water in various chemical and catalytic reactions as well as water in biological organisms where hydrogen bond plays an important role.
References
- [1] Y. Harada, T. Tokushima, Y. Horikawa, O. Takahashi, H. Niwa, M. Kobayashi, M. Oshima, Y. Senba, H. Ohashi, K. T. Wikfeldt, A. Nilsson, L. G. M. Pettersson, S. Shin, Phys. Rev. Lett. 111, 193001 (2013).
- [2] G. E. Walrafen, M. S. Hokmabadi, and W.-H. Yang, J. Chem. Phys. 85, 6964 (1986).
- [3] J.D. Smith, C. D. Cappa, K. R. Wilson, R. C. Cohen, P. L. Geissler, R. J. Saykally, Proc. National Acad. Sci. (USA) 102, 14171 (2005).
- [4] Y. Nagata, R.E. Pool, E.H.G. Backus, M. Bonn, Phys. Rev. Lett. 109, 226101 (2012).
