Interlayer Switching of Reduction in Layered Bi-V Oxide
Y. Ueda Group
The pseudo binary oxide system Bi2O3-V2O5 has received considerable interest due to its wide structural diversity and rich functional properties. Of particular interest is Aurivillius phase Bi4V2O11 which is found exhibiting remarkably high oxygen anionic mobility. Such a function promises serious potential applications in many important areas, e.g. solid oxide fuel/electrolysis cells and oxygen sensors. The crystal structure of idealized Bi4V2O11 is built up from infinite (Bi2O2)2+ sheets sandwiched between oxygen deficient VO4-Δ perovskite slabs (V-O layer). Intrinsic oxygen vacancies (Δ = 0.5) in the V-O layer, which is normally believed to enable high oxide ion conductivity, are located in both apical and equatorial sites of VO6 octahedra randomly. Such a description represents a widely accepted prototype structure for the high temperature γ-phase. With decreasing temperature, Bi4V2O11 undergoes two consecutive and reversible phase transitions, γ→β and β→α at 553 and 386 ºC, respectively, due to the long range oxygen vacancy ordering process.
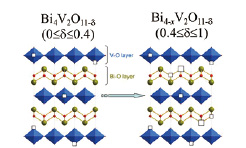
Fig. 1. Interlayer Switching of Reduction from V-O layers to Bi-O layers in Layered Oxide, Bi4V2O11-δ (0≤δ≤1). With increasing oxygen deficiency in Bi4V2O11-δ, the reduction of Bi4V2O11-δ first proceeds in the V-O layers, but beyond δ=0.4, suddenly switched to the Bi-O layers by precipitation of metallic bismuth, retaining a molar ratio of V4+/V5+ = 2/3, namely the relation between bismuth and oxygen deficiencies is expressed as x = 2δ/3 - 4/15 in Bi4-xV2O11-δ (0.4≤δ≤1).
The oxygen-vacancy-disordered γ-phase reminds us that there might exist a rich phase diagram in the oxygen deficient system where a wealth of unknown phases would be expected. Moreover, Bi4V2O11-δ is of particular interest as the two-dimensional square lattice enables Aurivillius a favoured structure carrier of novel quantum properties. A full phase diagram for the Bi4V2O11-δ (0≤δ≤1) system was built for the first time by examining the whole spectrum of composition [1]. One structure (α-phase) related to the established Bi4V2O11 phase survives in a very narrow δ range (≈ 0.1) whereas the other structure type (A-type) with its parent phase Bi4V2O10.6 shows unusual robustness from δ=0.4 until δ=1 (0.1<δ<0.4: two phase mixture). Surprisingly, the decreasing oxygen stoichiometry in this structural region is found to be accommodated not by lowering the valence of vanadium as would be expected, but instead by reducing bismuth. It means the Bi site is favored over the V site upon reduction. This conclusion was further strengthened by the measurements of magnetic susceptibility over different compositions. All samples (0.4≤δ≤1) show the similar behavior that is characteristic of a one dimensional S = ½ antiferromagnetic Heisenberg chain model. The rigid V-O unit shared by all compositions between δ=0.4 and 1 is thus responsible for the robustness of the second type structure in the current phase diagram. In situ XRD investigations as a function of temperature provided strong evidence for the existence of three allotropic forms, which are α, β and γ for the Bi4V2O11 type and A, B and γ for the Bi4V2O10.6 type. When heated to 570ºC or higher temperature, all compositions become uniform by forming the same tetragonal γ phase.
These results means that the reduction of Bi4V2O11-δ first proceeds in the V-O layer, but beyond δ=0.4, suddenly switched to the Bi-O layer by precipitation of metallic bismuth, retaining a molar ratio of V4+/V5+ = 2/3, namely the relation between bismuth and oxygen deficiencies is expressed as x = 2δ/3 - 4/15 in Bi4-xV2O11-δ (0.4≤δ≤1).
With a larger amount of intrinsic disordered oxygen vacancies, γ-Bi4V2O11-δ phases, particularly γ-Bi3.6V2O10 hold great potentials in offering optimized ionic conductivity. On the other hand, given the interesting defect chemistry and electromagnetic properties, this oxygen deficient system provides a very unique opportunity for the study at the interplay between structure and property.
References
- [1] Y. Zhang and Y. Ueda, Inorganic Chemistry 52, 5206 (2013).
