Giant Negative Thermal Expansion in SrCu3Fe4O12
I. Yamada and K. Ohgushi
We have synthesized a novel Fe4+-perovskite SrCu3Fe4O12 (SCFO) using high pressure synthesis method (15GPa, 1000 ºC) [1]. This compound consists of pseudo-square-coordinated Cu2+ ions and unusual high valence Fe4+ ions with octahedral coordination. Synchrotron powder X-ray diffraction (SXRD) data demonstrated a negative thermal expansion (NTE) in temperature range of 170–270 K (Fig. 1). The maximum value of the linear expansion coefficient (α) is α = –2.26×10–5 K–1 (Fig. 2(a)), which is comparable with the highest value (α = –2.5×10–5 K–1) for an antiperovskite nitride [2]. To our knowledge, this is the first observation of giant NTE in iron-based compounds.
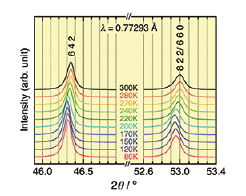
Fig. 1. SXRD patterns of SrCu3Fe4O12 in the vicinity of 6 4 2 and 8 2 2/ 6 6 0 Bragg reflections between 80 and 300 K.
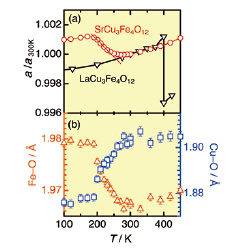
Fig. 2. (a) Temperature dependence of (a) the lattice constant normalized at 300 K (a/a300K) compared with that of LaCu3Fe4O12 [3] and (b) the metal–oxygen bond length.
Rietveld refinement based on the SXRD data indicates that the origin of the giant NTE in SCFO is a crossover-like intersite charge transfer between Cu and Fe. The bond valence sums (BVS) for Cu and Fe calculated from the metal–oxygen bond lengths (Fig. 2(b)) gradually change from +2.21 (Cu) and +3.54 (Fe) at 300 K to +2.99 (Cu) and +3.24 (Fe) at 80 K. The decreased nominal valence of Fe ion was confirmed by Mössbauer spectroscopy. The charge-disproportionated transition was observed below ~200 K with an abundance ratio of Fe3+: Fe5+ ≒ 4 : 1 at 4 K. This indicates that the nominal valence of Fe is ~+3.4 at ~200 K, as expected from the charge disproportionation transition of 5Fe3.4+ → Fe5+ + 4Fe3+.
The expansion of the lattice constant a on cooling in NTE temperature range is attributed to the elongation of Fe–O bond length (dFe–O) based on the crystallographic relation of a = 4dFe–O×sin(ψ/2), where ψ/2 is a Fe–O–Fe bond angle. The NTE behavior of SCFO is clearly distinguished from an abrupt volume expansion in LaCu3Fe4O12 (LCFO) [3] (Fig. 2(a)). This demonstrates the versatility of structural and electronic properties in the Fe4+-perovskite oxides. We are now studying A-site cation substitution effect of ACu3Fe4O12 perovskite further in order to clarify the relationship between crystal structure and electronic property.
References
- [1] I. Yamada, K. Tsuchida, K. Ohgushi, N. Hayashi, J. Kim, N. Tsuji, R. Takahashi, M. Matsushita, N. Nishiyama, T. Inoue, T. Irifune, K. Kato, M. Takata, and M. Takano, Angew. Chem., Int. Ed. 50, 6579 (2011).
- [2] K. Takenaka and H. Takagi, Appl. Phys. Lett. 87, 261902 (2005).
- [3] Y. W. Long, N. Hayashi, T. Saito, M. Azuma, S. Muranaka, and Y. Shimakawa, Nature 458, 60 (2009); W. Chen, Y. Long, T. Saito, J. P. Attfield, and Y. Shimakawa, J. Mater. Chem. 20, 7282 (2010).
