Coupled Electron-Proton Transfer Dynamics in Electrochemical Media
Sugino Group
Protons couple with water molecules in solution to form a hydronium ion (H3O+(aq)), which is sometimes regarded as a small polaron. At low voltages, instead, they prefer to combine with an electron near the electrode and are adsorbed thereon as a neutral species (H0(ads)). This is a prototypical electrochemical reaction called Volmer step. This change in the proton state is called coupled electron-proton transfer dynamics; its microscopic elucidation, however, has long been a challenge of theoreticians. A few decades ago, Schmickler modeled it by adding solvent phonons coupled harmonically with protons to a surface adsorption model called Newns-Anderson model. The resulting Newns-Anderson-Schmickler model, consisting of a hydrogen atom, electrons in electrode, and solution, has been regarded as one of the standard models for the electrochemical step.
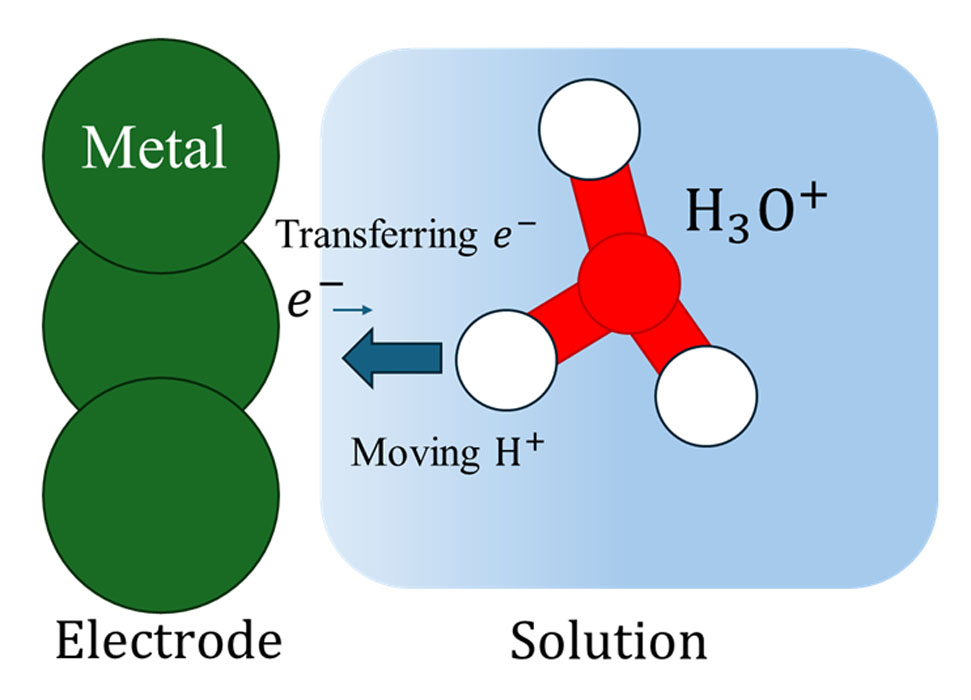
Fig. 1. Electrode-solution interface. Green circles represent the atoms constituting the electrode, and the red and while circles represent, respectively, the oxygen and hydrogen atoms constituting the hydronium ion. The hydrogen atom nearest to the electrode is moving towards the electrode, and this motion is coupled to transfer of an electron in the electrode. The Newns-Anderson-Schmickler model represent the coupled proton-electron transfer dynamics, for which an analytical solution has been provided in our research.
It is found that the terms added to consider the solvation effect can be apparently removed by applying the Lang-Firsov transformation, often used in studying polaron dynamics. This technique allows us to apply the non-equilibrium approaches so far developed for the Newns-Anderson model. Based on this recognition, we provided an analytical expression for the Volmer dynamics and revealed the non-adiabaticity caused by the moving hydrated protons, i.e. the deviation from the adiabatic Born-Oppenheimer (BO) state [1]. This is an extension of our previous work on the Volmer dynamics based on first-principles BO dynamic simulation [2] although the model used therein has been greatly simplified in the present Schmickler model. Importantly, however, it is possible for the model to consider the energy dissipation channels; (a) electron-hole excitation in the electrode or the electronic friction channel, and (b) vibrational excitation in the solvents. The former channel opens mainly when the proton affinity level aligns with the Fermi level and is increasingly important as the velocity of the proton increases, while the latter channel is constantly active under the conditions we have assumed. Our solution to the time-dependent News-Anderson-Schmickler model can be used to explain qualitatively the experiments regarding how the reaction rate depends on the electrode potential. The solution can also be used to show how the kinetic energy of the hydrated protons is dissipated into the dissipation channels, which should be important for the chemical-to-electric energy conversion efficiency. As far as we know, the two channels have not been studied together although each of them has been studied separately; the electrode problem thus provides a novel target for non-equilibrium statistical physicists.
Our simulation is currently based on assumed trajectories of the proton although the trajectory can be assumed only for fast-moving protons; in the present case, however, protons are moderately accelerated in the electric double layer, possibly on the order of several tens of meV. In the future step, therefore, the trajectory will be determined by solving the proton's equation of motion considering self-consistently the two dissipation channels as described above. We will consider in addition the quantum effect of protons, such as tunneling and interference, that may play a role as discussed in the literature. Those are the target of our long-term project “Establishing a Quantum Theory of Electrodes”. Our study has enabled us to take an important step in this direction.
References
- [1] E. F. Arguelles and O. Sugino, J. Chem. Phys. 160, 144102 (2024).
- [2] M. Otani, I. Hamada, O. Sugino, Y. Morikawa, Y. Okamoto, and T. Ikeshoji, J. Phys. Soc. Jpn. 77, 024802 (2008).
