Structural Study of Perovskite-type RbNbO3 Prepared at High Pressure
PI of Joint-use project: Ayako Yamamoto
Host lab: Yamaura group and X-Ray Diffraction Section
Host lab: Yamaura group and X-Ray Diffraction Section
We successfully synthesized a perovskite-type RbNbO3 compound by subjecting the non-perovskite RbNbO3 [1,2] to high temperature (1173 K) and high pressure (4 GPa) using a cubic-type high-pressure press. We investigated the temperature dependence of crystal structure associated with dielectric properties. Base on the powder X-ray diffraction patterns collected at ISSP under the collaborative program, we determined lattice parameters of the temperature dependence of perovskite RbNbO3 at 4–300 K. Our objective in this project is to discover novel ferroelectrics that are equal to or beyond BaTiO3, which is often utilized in a range of electrical devices. KNbO3 is a widely recognized displacement-type ferroelectric material, and we aimed to replace the potassium (K) ion with rubidium (Rb) ion, which belong to the same group of alkali metals but have a greater ionic radius. RbNbO3 has a complex quasi one-dimensional structure under normal conditions, unlike the perovskite-type structure found in KNbO3. We synthesized RbNbO3 with the perovskite structure using the high pressure technique. Detailed study was published in the reference [2].
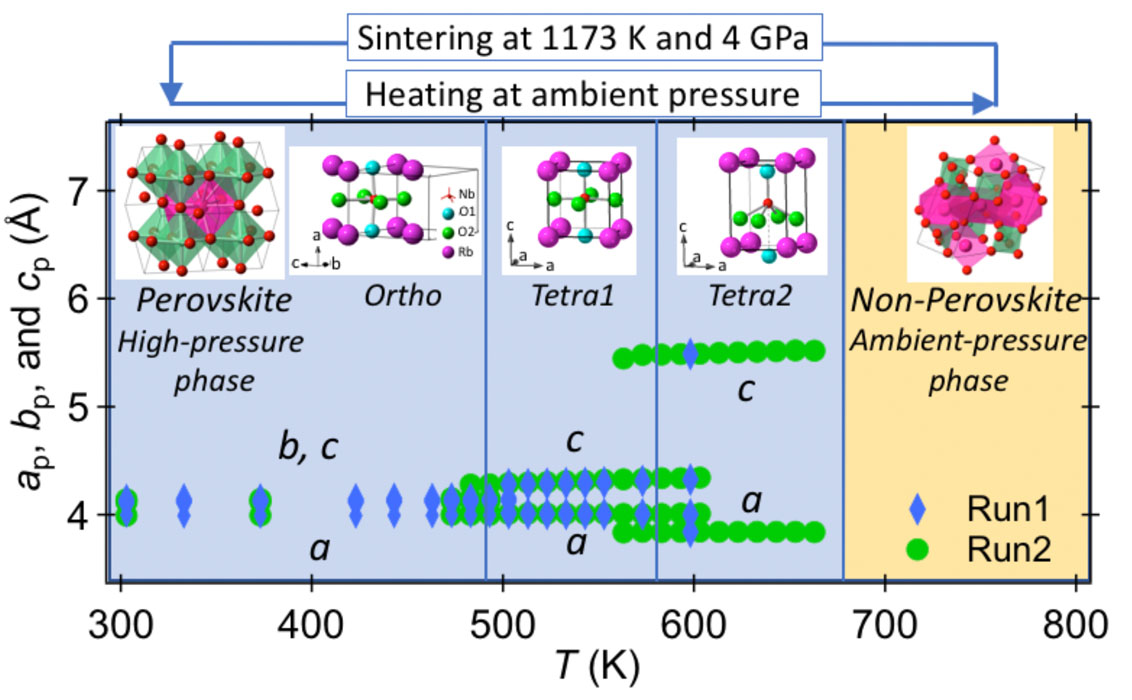
The high-pressure phase of RbNbO3 at 300 K was found to have an orthorhombic cell with a perovskite-type structure. The crystal structure was determined by single-crystal X-ray diffraction analysis at Tohoku University. The space group was identified as Amm2, with a lattice parameter of a = 3.9937(2) Å, b = 5.8217(3) Å, and c = 5.8647(2) Å. The non-centrosymmetric space group of RbNbO3 is same as that of the ferroelectric compounds BaTiO3 and KNbO3. The degree of distortion in RbNbO3 is more pronounced than in KNbO3, perhaps because of the larger ionic radius of Rb. The transitions from an orthorhombic structure to two sequential tetragonal phases (Tetra1 at 493 K, Tetra2 at 573 K) were observed. The perovskite framework was maintained during both phases before returning to the triclinic ambient phase at 693 K, as seen in Fig. 1. The first transition is similar to that observed in KNbO3, however the subsequent transition from the Tetra1 phase to the Tetra2 phase is distinct, characterized by elongation along the c-axis and a notable increase in the cp/ap ratio (cp and ap are taken with perovskite basic cell) from 1.07 to 1.43. This distortion indicates a transition that is comparable to the one observed in PbVO3 [3], where the oxygen atoms of an octahedron move apart along the c-axis, resulting in the formation of a pyramid as shown in Fig. 1. The permittivity exhibits a discontinuous increase at the orthorhombic to tetragonal phase transition, however this enhancement is not observed for the Tetra1 and Tetra2 transitions due to the collapse of the bulk sample caused by abrupt volume expansion during the transition.
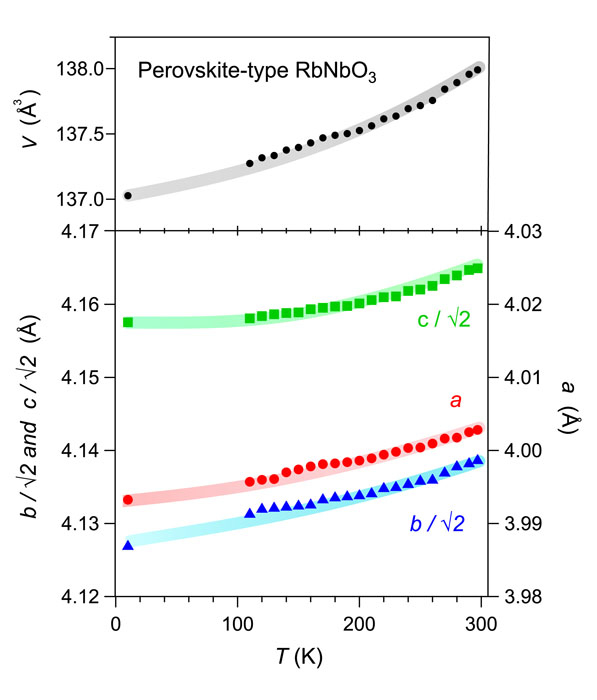
There were no observable alterations in the structure between temperatures of 4 and 300 K. The lattice parameters, a, b, c, and V exhibited consistent decreases with decreasing temperature, as seen in Fig. 2. A contrasting structural phase shift from orthorhombic to rhombohedral occurs at 220 K in KNbO3. This aligns with the findings reported by Fukuda et al. [1]. Nevertheless, the theoretical calculations regarding phase stability indicate that the orthorhombic structure with Amm2 is the most stable phase in RbNbO3, exhibiting the lowest energy [2]. It is contrast to that the stable space group in KNbO3 at low temperature is R3m.
In addition, we conducted thermal property measurements using a combination of thermogravimetry (TG), differential thermal analysis (DTA), and differential scanning calorimetry (DSC). Furthermore, we assessed the optical characteristics using second harmonic generation (SHG) [2]. We further synthesize a solid solution of RbNbO3 and KNbO3 by using the high pressure method, and investigate the structural studies with wide range of temperature. Ongoing research involves doing structural studies at various temperatures.
References
- [1] M. Fukuda and K. Yamaura, J. Ceram. Soc. Jpn. 131, 126 (2023).
- [2] A. Yamamoto, K. Murase, J. Yamaura, et al. Dalton Trans. 53, 7044 (2024).
- [3] R. V. Shpanchenko et al., Chem. Mater. 16, 3267 (2004).