Element-Specific Cluster Growth on the Two-Dimensional Metal–Organic Network
PI of Joint-use project: N. Tsukahara
Host lab: Yoshinobu Group
Host lab: Yoshinobu Group
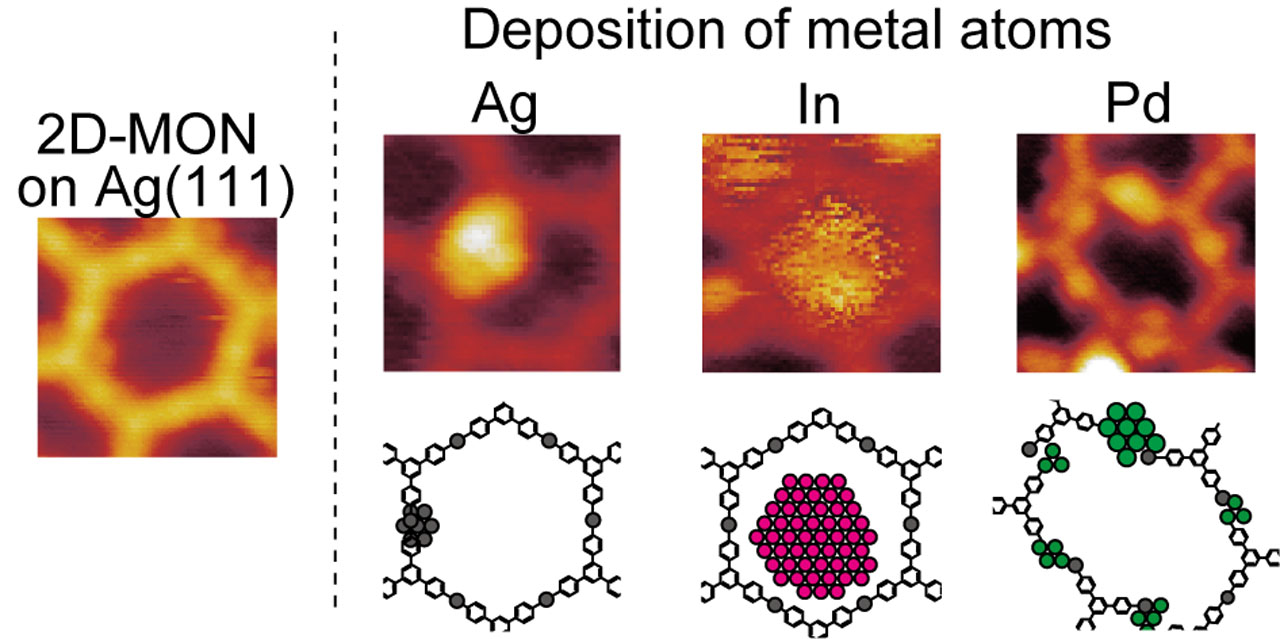
Solid surfaces provide a platform to fabricate various low-dimensional structures, and the formation of surface-supported two-dimensional metal–organic networks (2D-MONs) based on supramolecular chemistry is also achieved on the substrate. Porous two-dimensional metal–organic network (2D-MON) on a substrate could capture deposited metal atoms and metal clusters growing in the pores of the 2D-MON. Growth of a metal cluster in a pore of the 2D-MON requires that the pore is a local minimum of the potential energy surface, that is, a potential well for the metal adatoms. By arranging nanometer-scale potential wells on the substrate, deposited metal atoms form metal nanoclusters spontaneously.
We investigated the growth of Ag, In, and Pd nanoclusters in the 2D-MON by scanning tunneling microscopy (STM) measurements and density functional theory (DFT) calculations, and found that the growth mechanisms of Ag, In, and Pd clusters in the 2D-MON synthesized from 1,3,5-tris(4-bromophenyl) benzene molecules on Ag(111) are different from each other. From the STM measurement, Ag and Pd clusters grow from the 2D-MON, especially two-coordinated Ag atoms in the 2D-MON. Indium clusters grow in the center of pores. Based on our DFT calculations, the total energy of an adatom in a pore depends on the position of the adatom, and the interaction of Ag and Pd adatoms with the 2D-MON is attractive. On the other hand, the interaction between an In adatom and the 2D-MON is repulsive. Since the net-charges of Ag, In, Pd adatoms on Cu(111), and two-coordinated Ag in the 2D-MON are −0.01e, 0.38e, and −0.14e, and 0.23e, respectively, the electrostatic interaction between In and Pd adatoms and the 2D-MON may play a significant role. For Ag, the DFT calculation without van-der Waals correction results in repulsive interaction with the 2D-MON. We think that van-der Waals interaction plays an important role in the Ag nanocluster formation in the 2D-MON. The growth process of metal clusters is determined by the element-specific behavior of metal adatoms in the pores, taking into account the various interactions with the 2D-MON.
References
- [1] N. Tsukahara, R. Arafune, and J. Yoshinobu, Jpn. J. Appl. Phys. 63, 065504 (2024).