Dynamics of Acetonitrile Absorbed in a Metal–Organic Framework MIL-101 with Mg-Ion Conduction
Yamamuro Group
MIL-101 is a kind of Metal–Organic Framework (MOF), which contains numerous pores and attracts great attention for various applications, e.g., gas reservoirs, filters, reaction fields, ionic conductors, etc. Recently, the group of Prof. Sadakiyo (our collaborator and visiting professor of ISSP) discovered that MIL-101 containing Mg(TFSI)2 (TFSI: Bis(trifluoromethanesulfonyl)imide) exhibits superionic conductivity of around 10-3 S cm-1 after absorbing acetonitrile (AN) vapor [1]. This discovery may contribute to the development of Mg-ion batteries, which are promising candidates for non-lithium-ion solid-state batteries.
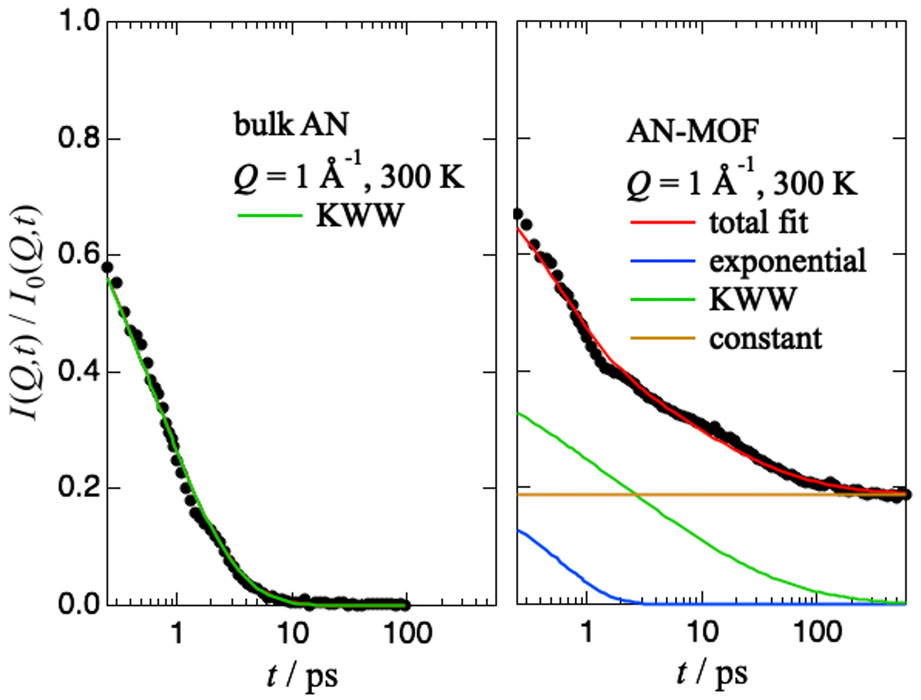
To clarify the effect of AN on Mg-ion conduction, we conducted a quasielastic neutron scattering (QENS) experiment using AGNES at JRR-3 and DNA at J-PARC for three samples: bulk AN, MIL-101 with absorbed AN (AN-MOF) and MIL-101 with absorbed AN and Mg(TFSI)2 (AN-Mg-MOF). The QENS spectra observed by AGNES and DNA were Fourier transformed into intermediate scattering functions I(Q,t)s and smoothly joined as shown in Fig. 1. As shown in Fig. 2, the I(Q,t) of bulk AN is fitted well by a single Kohlrausch-Williams-Watts (KWW) function as ordinary α relaxations of molecular liquids, while those of AN-MOF and AN-Mg-MOF by the sum of a KWW function, a constant (elastic component) which corresponds to the stational hydrogen atoms in MIL-101, and an exponential function which may correspond to a methyl rotation decoupled from the α-relaxation.
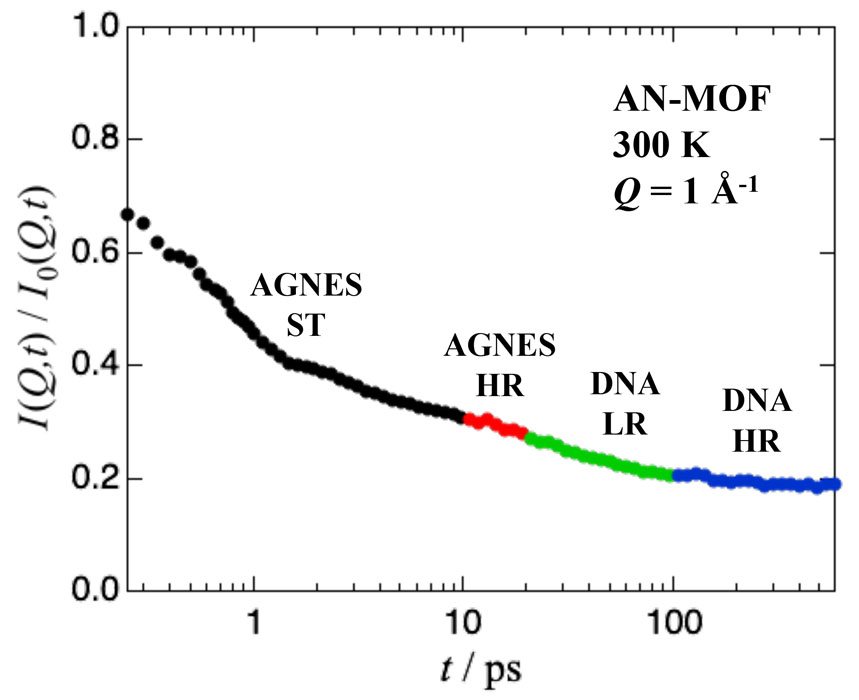
Fig. 1. Intermediate scattering functions of AN-MOF obtained from the Fourier transformation of the QENS data at T = 300 K and Q = 1 Å-1. The I(Q,t) data from the standard (ST) and high-resolution (HR) modes of AGNES and the low-resolution (LR) and high-resolution (HR) modes of DNA are smoothly joined.
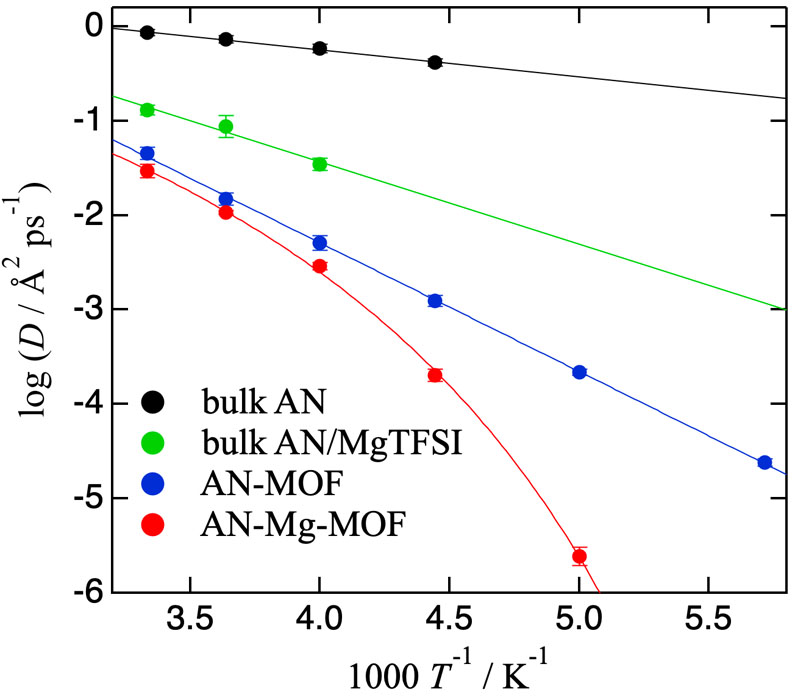
To investigate the α-relaxation expressed by the KWW function, we examine the Q2-dependence of the inverse of the average relaxation time <τ>-1. The plots for bulk AN and AN-MOF show continuous diffusion (<τ>-1 = DQ2, D: diffusion constant), whereas that for AN-Mg-MOF is represented by the jump-diffusion model (<τ>-1 = DQ2/(1 + τ0DQ2), τ0: mean residence time). This jump may be related to the connection/disconnection process of AN molecules to Mg2+. Figure 3 displays the Arrhenius plots of D comparing the three samples. The plots for bulk AN and AN-MOF are classified to Arrhenius type, while that for AN-Mg-MOF is non-Arrhenius type. This may be because AN molecules coordinate to Mg2+ ions and the number of the coordinated AN molecules increases as temperature is lowered. The diffusion constants become smaller in order of bulk AN, AN-MOF and AN-Mg-MOF, indicating that the diffusion of AN is hindered by the pore walls of MIL-101 and further by Mg2+ ions. The mean jump distance <l> was calculated by <l> = (6Dτ0)1/2 to be 1.6 Å. The results shown above should provide important information to clarify the mechanism of Mg2+ conduction.
References
- [1] Y. Yoshida et al., J. Am. Chem. Soc. 144, 8669 (2022).