Mechanical Conformation Control of Cyclized Binaphthyl at the Air–Water Interface
PI of Joint-use project: K. Ariga
Host lab: Lippmaa Group
Host lab: Lippmaa Group
Biological activity is maintained by various molecular machines working in the body. The complex structure of molecular machines makes it difficult to elucidate their behavior. Molecular machines function through a combination of various molecular conformation changes. Detailed analysis of simple molecular conformation changes is expected to provide a deeper understanding of the operating mechanisms of complex molecular machines. The air-water interface is a suitable field for controlling molecular machines because mechanical compression can be applied with the help of movable barriers of the Langmuir–Blodgett (LB) system, comparable to those that can change the conformation of proteins. In addition, a lipid matrix, which is a medium analogous to biological membranes, can be prepared at the air-water interface.
Molecular conformations can be identified using several spectroscopic methods. We have confirmed the conformational change of binaphthyl derivatives at the air-water interface. The circular dichroism (CD) spectra can be obtained for these molecules at low concentrations and the spectra sensitively reflect conformational changes [1, 2]. In this work, we have controlled the molecular conformations of monobinaphthyldurene (MBD) in a lipid matrix similar to biological systems [3].
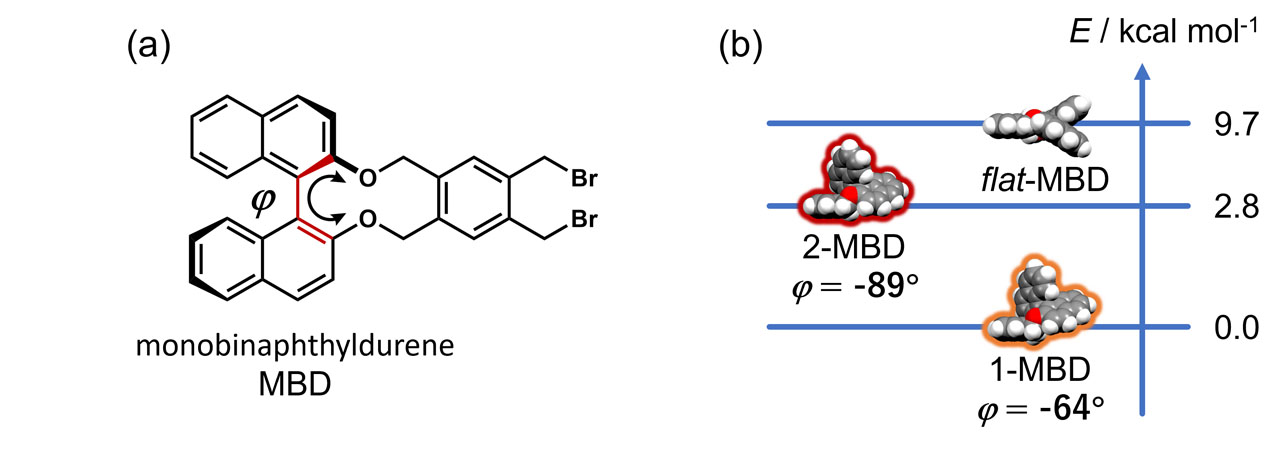
Figure 1 shows the chemical structure of MBD and three conformers of 1-MBD, 2-MBD, and flat-MBD, which is indicated by density functional theory (DFT) calculations, having different dihedral angles of φ between the two naphthyl moieties. The conformer 2-MBD is 2.8 kcal mol–1 higher in energy than the more stable 1-MBD, while flat-MBD is not favored energetically. DFT calculations also indicate that 2-MBD has a larger dihedral angle than 1-MBD, which causes the CD peak of 2-MBD to shift to a longer wavelength.
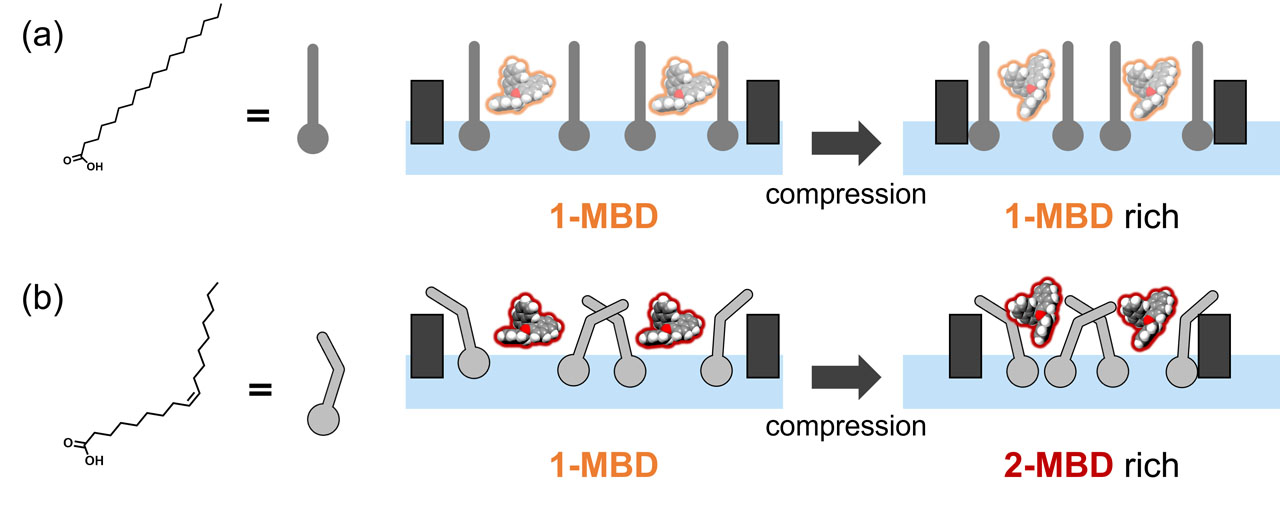
MBD was mixed with linear stearic acid (SA) or unsaturated oleic acid (OA) as matrices and then spread at the air–water interface. The mechanical properties of the mixed monolayers with MBD were characterized by surface pressure–molecular area (π-A) isotherms. The monolayers were transferred onto quartz substrates at different surface pressures to analyze the molecular aggregations and conformations by using CD spectra and Fourier transform infrared reflection absorption spectroscopy (FT-IR-RAS), atomic force microscopy (AFM). It suggested that MBD mixes more effectively with OA than with SA and dissolves in the lipid matrices upon mechanical compression.
The π-A isotherms suggest high miscibility of MBD with OA. It was also supported by FT-IR-RAS and AFM. The CD peak of the mixed monolayer with SA did not change with surface pressure. On the other hand, the mixed monolayer with OA shifted to longer wavelength with increasing surface pressure. Thus, the conformation of MBD changes from 1-MBD to 2-MBD in the mixed monolayer of OA; in contrast, 1-MBD is stable in SA. The higher-energy conformer 2-MBD is stabilized by the dissolution in the highly miscible lipids of OA.
As summarized in Figure 2, MBD confirmations could be controlled from 1-MBD to 2-MBD by mechanical stimulus in highly miscible lipids of OA. In contrast, the most stable 1-MBD conformation could not be transformed during mechanical compression in poorly miscible lipids of SA. It was found that different behaviors of molecular machines showed in their different local environments.
References
- [1] D. Ishikawa, T. Mori, Y. Yonamine, W. Nakanishi, D. L. Cheung, J. P. Hill, and K. Ariga, Angew. Chem. Int. Ed. 54, 8988 (2015).
- [2] M. Ishii, T. Mori, W. Nakanishi, J. P. Hill, H. Sakai, and K. Ariga, ACS Nano 14, 13294 (2020).
- [3] M. Ishii, T. Mori, W. Nakanishi, J. P. Hill, H. Sakai, and K. Ariga, Langmuir 38, 6481 (2022).