Rotations of Methane Molecules in Amorphous and Crystalline Hydrates
Yamamuro Group
Methane hydrate has been studied extensively not only as a future energy resource but also from the interest in quantum and classical rotations of methane molecules. The crystalline methane hydrate (c-MH) forms a host water lattice of type I with two dodecahedral (12-hedral) and six tetradecahedral (14-hedral) cages per unit cell (space group: Pm3n), and each cage contains one methane molecule [1]. The chemical formula of c-MH is CH4・5.75H2O. The “amorphous” MH (a-MH) was first prepared with a vapor-deposition method by our group [2]. The radial distribution functions derived from the neutron diffraction data demonstrated that the methane molecules are accommodated in the cage-like spaces even in amorphous structures. It is also known that the a-MH crystallizes into the c-MH around 175 K [3].
In this study, we have investigated the rotations of methane molecules in a-MH and c-MH. Our main interest is how the rotation of methane molecules is affected by the cage (like) structure, e.g., size and symmetry. The a-MH was prepared by depositing the mixture of CH4 and D2O gases onto the substrate cooled at 5 K using a custom-made cryostat [4], and c-MH was obtained by annealing the sample at 170 K under 0.1 MPa of CH4 gas. Their quasi-elastic neutron scattering (QENS) and inelastic neutron scattering (INS) data were collected at 5 to 80 K using AGNES spectrometer at JRR-3, Tokai, Japan.
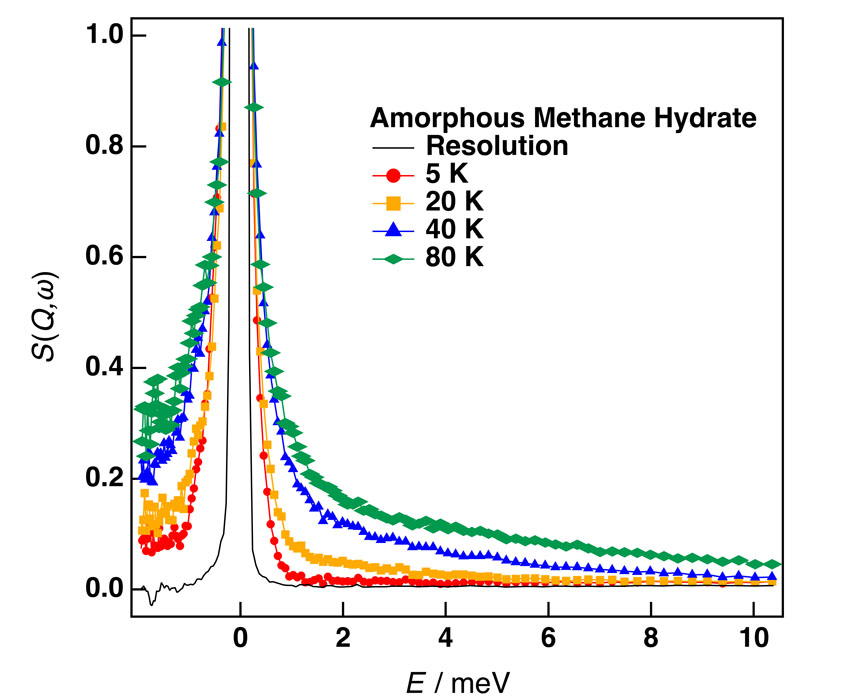
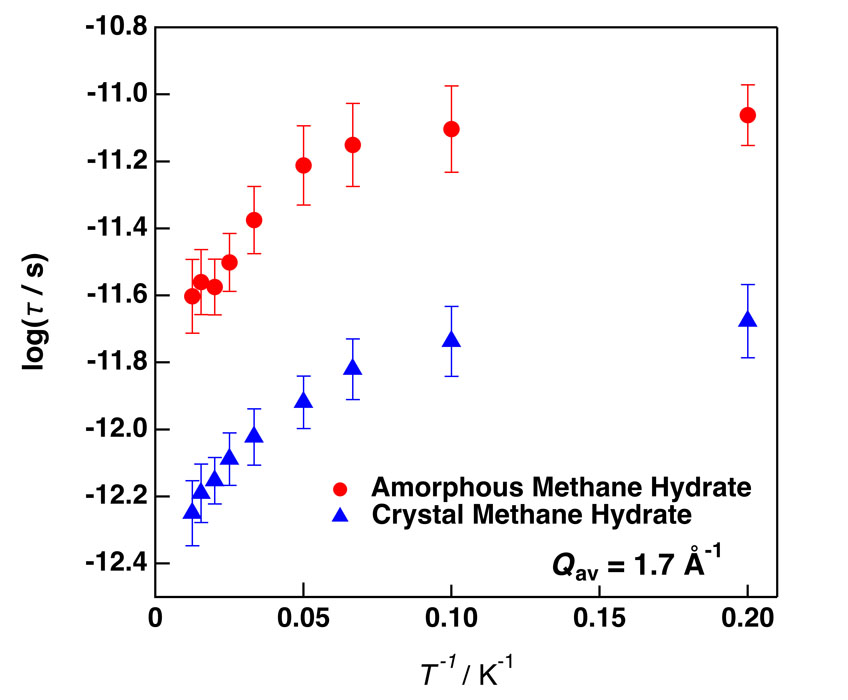
Figure 1 shows clear QENS broadening due to the rotational motion of CH4 accommodated in a-MH. It is worth noting that the QENS appeared even at 5 K. The data were well fitted to the combination of Delta and Lorentz functions as usual and the relaxation time τ was calculated from the peak width of the Lorentz function. Similar QENS data were observed also in c-MH. Figure 2 shows the Arrhenius plots for both a-MH and c-MH. There is a clear bending of the τ line around 20 K for both samples and the slope (activation energy ΔEa) on the low-temperature side is very small (ΔEa < 0.2 kJ/mol). We guess that there is a crossover between the classical (high-T) and quantum (low-T) rotations around 20 K; there is no temperature dependence of τ in a pure tunneling process. The a-MH has longer τ than that of c-MH. This is probably because the cage space in a-MH is narrower than that of c-MH. Additional neutron diffraction and calorimetric experiments are now going on to explain the QENS data more quantitatively.
References
- [1] D. W. Davidson et al., Nature 311, 142 (1984).
- [2] T. Kikuchi et al., J. Phys. Soc. Jpn. 81, 094604 (2012).
- [3] M. Z. Faizullin et al., Chem. Eng. Sci. 130, 135 (2015).
- [4] O. Yamamuro et al., J. Chem. Phys. 115, 9808 (2001).