Study on the Channel Gating Mechanism of Channelrhodopsin C1C2
Akiyama and Inoue Groups
Channelrhodopsins (ChRs) are members of microbial rhodopsins that function as light-gated cation channels. ChRs are composed of seven transmembrane (TM) helices and an all-trans-retinal chromophore which is covalently bound to the seventh TM helix via a protonated Schiff base linkage. Light absorption induces the isomerization of the retinal chromophore from the all-trans to the 13-cis form leading to the subsequent conformation change of the protein moiety opening the ion-transport channel connecting the extracellular and cytoplasmic solvents. Recently, ChRs are being used widely in optogenetics to control neural activity in an animal body. Whereas high-functional ChR proteins are being strongly demanded to realize efficient and low-invasive optogenetics, the lack of understanding of its channel-gating mechanism is inhibiting rational and precise protein engineering to improve the functionality of ChRs. The structural information of the open state of ChR is indispensable to clarify the mechanism. However, it was difficult due to the inhibition of the channel opening by the tight packing of protein molecules in the crystal phase for the X-ray crystallographic analysis.
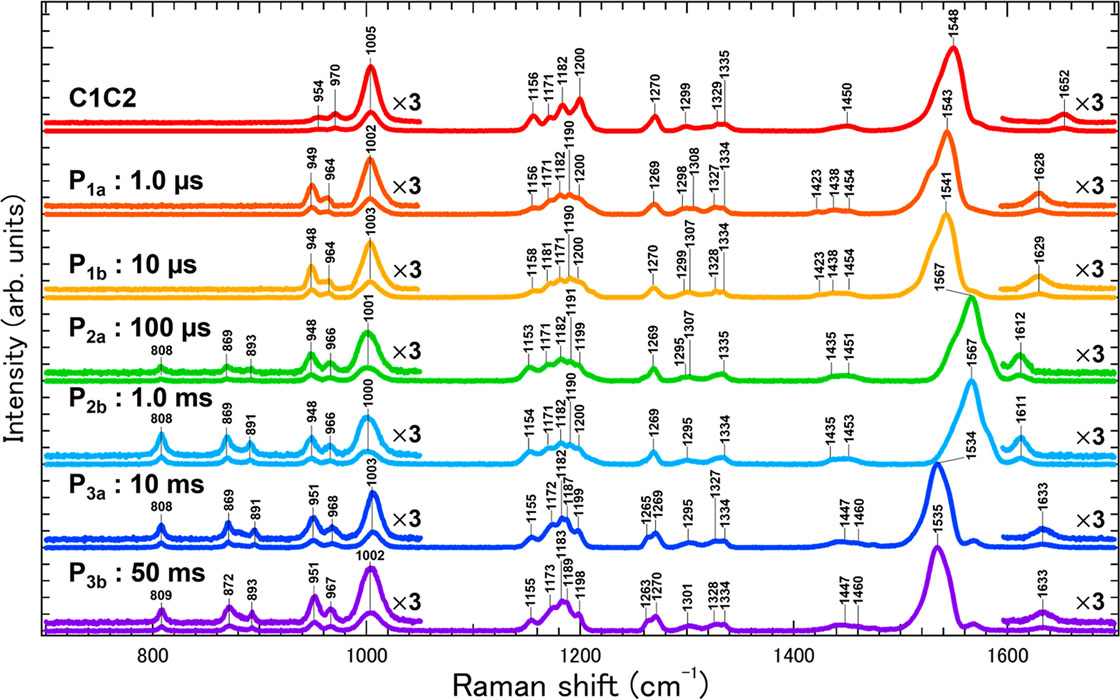
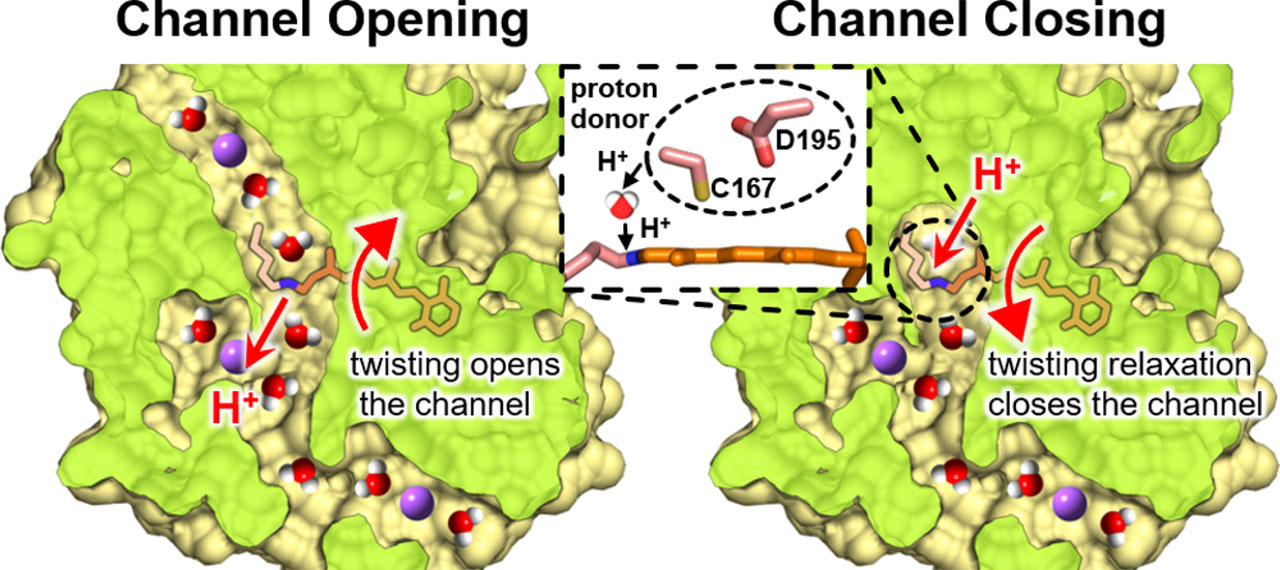
To overcome this difficulty, we studied the channel opening and closing processes of C1C2, one of the best characterized ChRs whose three-dimensional structure was first solved, by time-resolved resonance Raman spectroscopy, laser flash photolysis, and laser electrophysiology. Especially, time-resolved resonance Raman spectroscopy can selectively obtain detailed information on the chemical bonds of the retinal chromophore based on its vibrational modes. However, time-resolved resonance Raman spectroscopy generally requires a large amount of protein sample which is difficult to prepare for C1C2. We avoided this problem by developing a new Raman microspectroscopy system using semiconductor lasers, acoustic-optics modulators (AOM), an optical objective lens, and an automated optical stage. As a result, we succeeded in obtaining the resonance Raman spectra of the initial and all photointermediate states of C1C2 and revealed the enhancement of the hydrogen-out-of-plain mode of the retinal chromophore after the photoexcitation of C1C2 indicating the polyene chain of the retinal is more twisted compared with the dark state. The twisting of the retinal is maximized in the full-open state of C1C2. Hence, the channel gate in C1C2 is opened by the steric repulsion between the retinal chromophore and transmembrane helices as previously suggested by the quantum-mechanics/molecular-mechanics calculation [1] and time-resolved X-ray crystallographic study [2] on the pre-opened state. On the other hand, the shift and broadening of the C=N stretching vibration band revealed the switching of the hydrogen-bonding partner of the retinal Schiff base from the counterion to a water molecule during the channel opening. Furthermore, the laser flash photolysis and laser electrophysiology measurements revealed that, while the channel opening kinetics did not alter between H2O and D2O solvents, the rate of channel closing was significantly slowed in D2O compared to H2O. This result indicates that the protonation of the retinal chromophore relaxes the twisted polyene chain leading to the channel closing.
This study provided a new understanding of the channel gating mechanism of ChR in which the channel opening is induced by the retinal twisting and the closing rate is regulated by the proton transfer between the protein moiety and the chromophore [3]. The development of novel functional ChRs based on this understanding in the near future is highly expected.
References
- [1] C. Cheng, M. Kamiya, M. Takemoto, R. Ishitani, O. Nureki, N. Yoshida, and S. Hayashi, Biophys. J. 115, 1281 (2018).
- [2] K. Oda, T. Nomura, T. Nakane, K. Yamashita, K. Inoue, S. Ito, J. Vierock, K. Hirata, A. D. Maturana, K. Katayama, T. Ikuta, I. Ishigami, T. Izume, R. Umeda, R. Eguma, S. Oishi, G. Kasuya, T. Kato, T. Kusakizako, W. Shihoya, H. Shimada, T. Takatsuji, M. Takemoto, R. Taniguchi, A. Tomita, R. Nakamura, M. Fukuda, H. Miyauchi, Y. Lee, E. Nango, R. Tanaka, T. Tanaka, M. Sugahara, T. Kimura, T. Shimamura, T. Fujiwara, Y. Yamanaka, S. Owada, Y. Joti, K. Tono, R. Ishitani, S. Hayashi, H. Kandori, P. Hegemann, S. Iwata, M. Kubo, T. Nishizawa, and O. Nureki, eLife 10, e6238 (2021).
- [3] K. Shibata, K. Oda, T. Nishizawa, Y. Hazama, R. Ono, S. Takaramoto, R. Bagherzadeh, H. Yawo, O. Nureki, K. Inoue, and H. Akiyama, J. Am. Chem. Soc., doi: 10.1021/jacs.3c01879 (2023).