Thought Experiment of the Activity of an Oxide Electrode
Sugino Group
While a great deal of attention has been given to the computational design of functional materials, the degree of freedom is overwhelming when dealing with the heterogeneous interfaces of defective materials. Typical example is the defective oxides interfaced with solutions used for the electrocatalysis application. Recent experiments show that both zirconia and titania cathodes provide enhanced electromotive force of fuel cells when they contain nitrogen impurities and oxygen vacancies, but the microscopic structure of the electrode-electrolyte interface is not well understood. In this context, we use the large supercomputers such as Fugaku and the recently developed programs such as abICS [1] to perform ab initio Monte Carlo (MC) and molecular dynamics simulations to model the interface by sampling unprecedentedly large degrees of freedom. Note that abICS does not simply generate the ab initio trajectories, but also help enhance the sampling by machine learning the generated trajectories to significantly speed up the simulation. We used them to address the problem of understanding the reason for the improved fuel cell efficiency. In our approach, we first simulated the zirconia surface and then the zirconia-water interface.
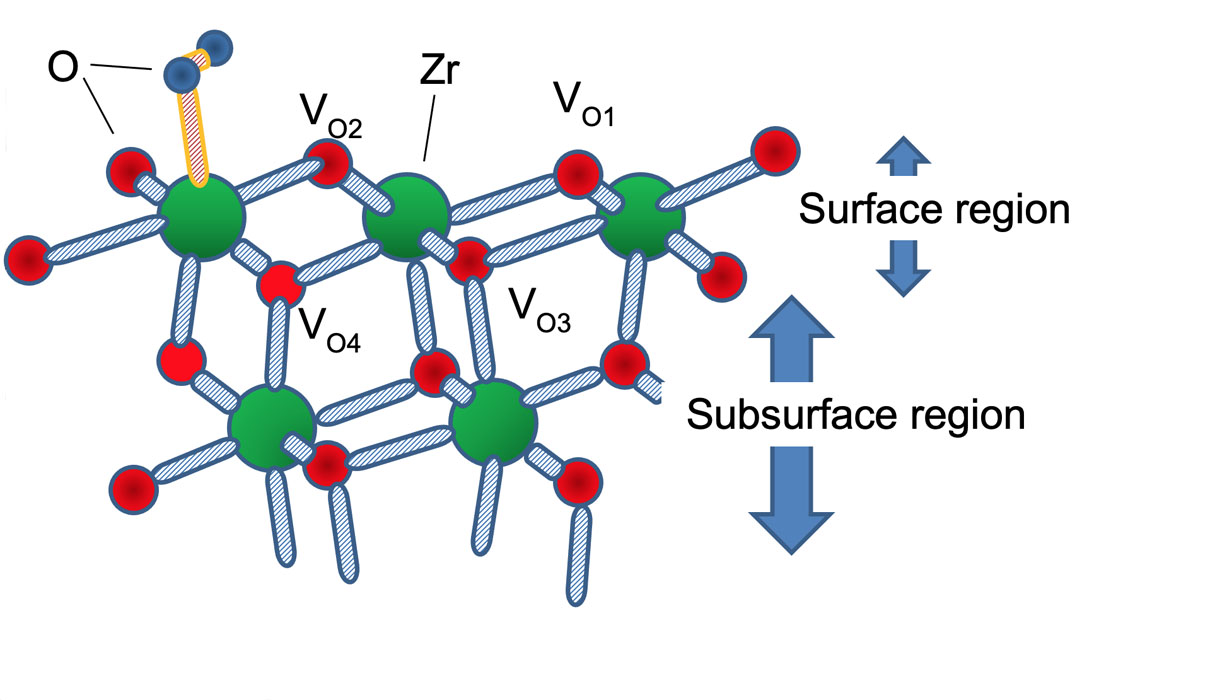
Fig. 1. (Schematic view) Part of the 2 × 1 slab model of the defective tetragonal ZrO2(101) surface where one O2 molecule (blue spheres) is adsorbed on a surface Zr atom (green) surface. We have examined all possible paths for the oxygen reduction reaction where the adsorbed O2 is reduced to form a water molecule. The defect site was modeled by removing a surface O atom, VO1 and VO2, and substituting a subsurface O atom with a N impurity.
Our previous simulation has revealed that an oxygen vacancy can be stabilized when doped with two times denser nitrogen impurities because of the charge compensation effect. The surface simulation showed that the vacancies are distributed not only at the surface but also in the subsurface regions, while the nitrogen impurities do not favor the surface sites. Based on this result, we reduced the size of the model surface so that we can examine all possible configurations of the ORR intermediates, i.e., O2*, O2H*, OH*, and O*, where * means adsorbed species. The resulting adsorption energies were used to obtain the free energy profile. The result showed that an oxygen molecule can be efficiently reduced to form water molecules on the vacancy site, with the activity comparable to that of the platinum electrode [2]. The activity was estimated thermodynamically using the empirical assumption that the barrier existing between the reaction intermediates does not play a role. Importantly, the estimated activity was found to be comparable to that of the non-defective surface. This result should be interpreted with caution. Considering the low availability of the active defects, we can conclude that the overall rate of ORR is likely to be higher for the non-defective surface, although we cannot rule out the possibility that we have missed important configurations due to the finite cell size and time used for the simulation. The apparent inconsistency with experiments that the efficiency of the fuel cell is increased by introducing the defects can be explained if the carrier supply is significantly increased by doping. This will be the case if defect induced gap states are extended in the sample. With this in mind, we are now theoretically investigating the electrostatic potential profile to see how the carrier path can be facilitated.
In the above surface simulation, we have neglected the solvation effect. Water molecules can be adsorbed on the zirconia surface as a molecule or as dissociated species, OH* and H*, blocking the incoming oxygen molecule to be adsorbed. This possibility was investigated using the machine-learned potential developed with abICS. Based on many nanosecond trajectories, we found that water molecules do not favor the surface vacancy site, while they favor reactive Zr sites when no defects are introduced. This is likely to be an indirect indication that the blocking effect is significantly different between the defective and pristine surfaces. Further simulations are now in progress to make a conclusion about the solvation effect.
References
- [1] https://www.pasums.issp.u-tokyo.ac.jp/abics/
- [2] S. Muhammady, J. Haruyama, S. Kasamatsu, and O. Sugino, J. Phys. Chem. C126, 15662 (2022).
