Elucidation of the Atomic-Scale Processes of Dissociative Adsorption and Spillover of Hydrogen on the Single Atom Alloy Catalyst Pd/Cu(111)
Yoshinobu and Ozaki Groups
The utilization of hydrogen in industry has become more important for our modern society because many indispensable processes consist of chemical reactions involving hydrogen: synthesis of chemical feedstocks such as methanol and ammonia, petroleum refining, energy sources through fuel cell systems, and so forth. In these reactions, dissociation of molecular hydrogen is an essential elementary step in hydrogenation reactions, where the active sites for hydrogen dissociation are necessary. In recent years, single atom alloy catalysts (SAACs) have been attracting much attention due to their excellent properties, such as high activity/selectivity and resistance to deactivation via coke formation or CO poisoning. In SAACs, active metal atoms are highly diluted and atomically dispersed on an inert but selective metal surface. Several previous studies have shown that the Pd/Cu SAAC shows excellent activity for hydrogen dissociation and hydrogenation reactions. On the Pd/Cu SAACs, the hydrogen spillover from Pd sites onto Cu host surfaces is a vital process for selective hydrogenation because it has been considered that the reaction proceeds on the Cu surface which weakly interacts with the product molecules.
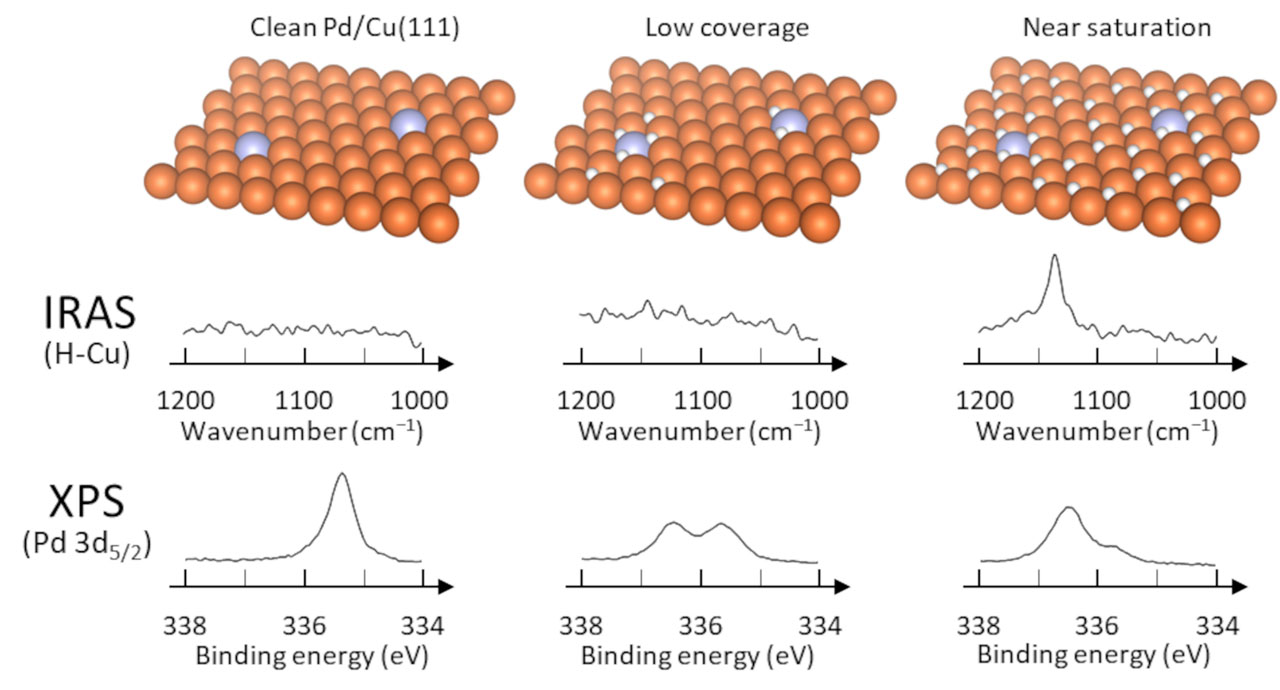
We investigated the dissociative adsorption of hydrogen and the subsequent spillover process on the Pd/Cu(111) surface using infrared reflection absorption spectroscopy (IRAS), temperature programmed desorption (TPD), and high-resolution X-ray photoelectron spectroscopy (HR-XPS), as well as density functional (DFT) calculations [1]. The hydrogen spillover onto Cu(111) was successfully observed in real time using time-resolved IRAS measurements. The chemical shifts of Pd 3d5/2 indicate that molecular hydrogen is dissociated and adsorbed at the Pd site. In addition, “two-step” chemical shifts of the Pd 3d5/2 binding energy were observed as a function of hydrogen exposure. The DFT calculation revealed that the “two-step” chemical shifts originate from the charge transfer (CT) from Pd to hydrogen; initially hydrogen atoms are directly adsorbed at the Pd site, and thereafter a significant amount of additional hydrogen atoms are adsorbed at the Cu sites in which CT occurs in an indirect manner. Based on the present experimental and theoretical results, we propose the following mechanism of the hydrogen dissociation and spillover process on Pd-Cu(111) : (i) a hydrogen molecule is dissociated at a Pd site, and the hydrogen atoms are adsorbed on the Pd site, (ii) the number of hydrogen atoms on the Pd site increases up to three, and (iii) the additional hydrogen atoms spill over onto the Cu surface.
References
- [1] W. Osada, S. Tanaka, K. Mukai, M. Kawamura, Y.H. Choi, F. Ozaki, T. Ozaki, and Jun Yoshinobu, Phys. Chem. Chem. Phys. 24, 21705 (2022).