Structure of Glass-Forming Molecular Liquids under High Pressure
Yamamuro Group
Glass transition is one of the most important unsolved problems in condensed matter physics. The history of the glass transition study might start when Giauque found a heat capacity jump of glycerol in 1923 [1]. After that many people have studied the mechanism of the glass transition both through experimental and theoretical approaches. High pressure studies have played important roles in the glass transition research since they provide direct information for the validity of the two important concepts of the mechanism of the glass transition; i.e., the entropy theory and free-volume theory. At present, the entropy theory, particularly Adam-Gibbs (AG) theory [2], is dominant to explain thermodynamic properties of the glass transition [3] and the pressure dependence of the glass transition temperature (Tg) [4]. An extended version of the AG theory, random first-order transition (RFOT) theory [5], can also explain the non-linear dynamics of the glass transition. Molecular liquids are important materials in the glass transition research since they are similar to the systems treated in theoretical and computer simulation studies. We are interested in van der Waals molecules which are condensed only by the van der Waals interaction, and hydrogen-bonding molecules which have network structures formed by intermolecular hydrogen bonds.
Recently, we have measured X-ray diffraction (XRD) data of toluene (C6H5CH3, Tg = 117 K) and glycerol [(CH2(OH)CH(OH)CH2(OH), Tg = 187 K] under high pressures (toluene: < 0.9 GPa, glycerol: < 3.6 GPa) at RT. This work is a collaboration with Prof. Yoshio Kono (Ehime Univ.). We used a Paris-Edinburg cell and BL37XU, SPring-8, on which very precise horizontal focusing and collimation are available.
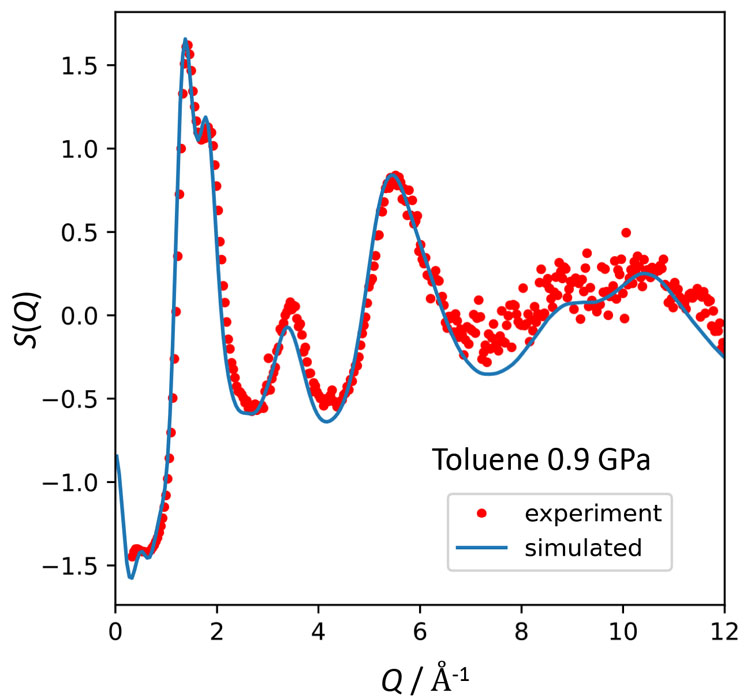
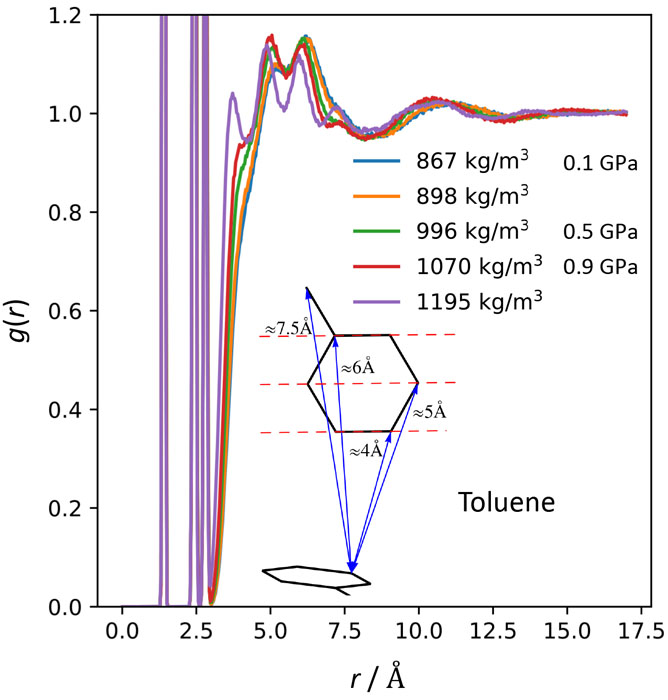
Figure 1 shows the structure factors of toluene at 0.9 GPa. The result of the MD simulation (AMBER force field, 0.2 ns, 500 molecules) is also shown. The MD simulation well reproduces the experimental data. Good agreement was obtained also for the data at 0 and 0.4 GPa. Figure 2 shows the pair distribution functions g(r) of aromatic (benzene-ring) carbons obtained by the MD simulations. As the density (pressure) increases, three peaks between 3 and 6 Å become more prominent. These peaks are known to be due to the T-type pair correlation as shown in the inset. It is noteworthy that this trend is the same as the cooling effect at ambient pressure. Hence, we concluded that the AG concept is valid also for the glass transition by pressurization.
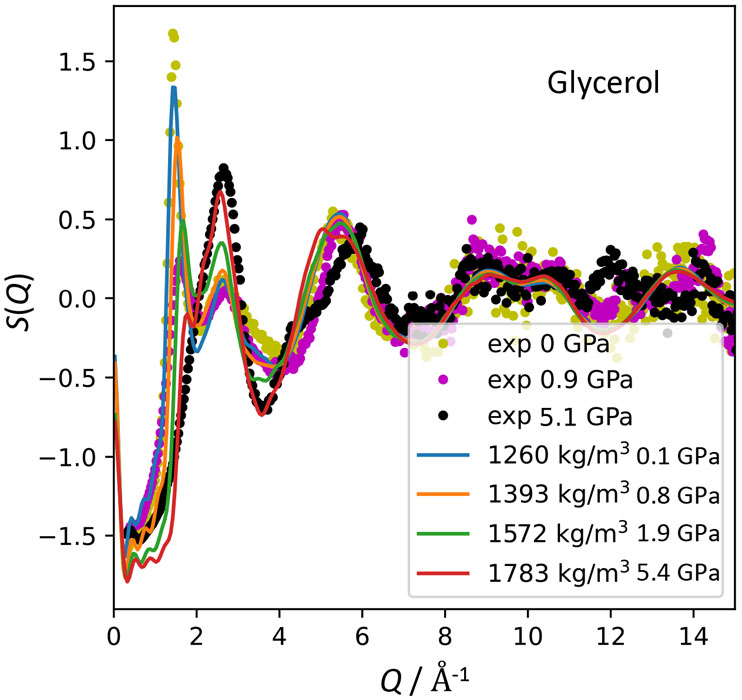
On the other hand, the structure factor of glycerol cannot be reproduced by the MD simulation using the potential parameters at ambient pressure. Therefore, we performed ab initio (Car-Parrinello) MD simulations using a smaller system (50 molecules) and shorter simulation time (0.15 ps) to determine the short-range structure, and then adjusted the Lenard-Jones parameters as a function of pressure so as to reproduce the g(r) obtained by the ab initio MD. Figure 3 shows the structure factors using the adjusted potential parameters. Good agreement is obtained between the experimental and MD data. Form the obtained structure, we found that the molecular structure is deformed to be more compact keeping hydrogen bonds. The C-O intermolecular correlation is also important under high pressure. The present conclusion is quite different from the common sense that hydrogen bonds are just broken by pressurization. A neutron diffraction experiment is under planning to determine the position of the hydrogen atoms and make clearer discussion on the competing hydrogen-bond and van der Waals interactions under high pressure.
References
- [1] G. E. Gibson and W. F. Giauque, J. Am. Chem. Soc. 40, 93 (1923).
- [2] G. Adam and J. H. Gibbs, J. Chem. Phys. 43, 139 (1965).
- [3] S. Tatsumi et al., Phys. Rev. Lett. 109, 045701 (2012).
- [4] O. Yamamuro et al., J. Non-Cryst. Solids, 183, 144 (1995).
- [5] T. R. Kirkpatrick et al., Phys. Rev. A 40, 1045 (1989).