Functionalization of the MoS2 Basal Plane for Activation of Molecular Hydrogen by Pd Deposition Studied by Ambient-Pressure X-ray Photoelectron Spectroscopy
Yoshinobu and I. Matsuda Groups
Molybdenum disulfide (MoS2) is one of the transition-metal dichalcogenides (TMDs) and has a layered structure. The monolayer or multilayer MoS2 is expected to have various applications such as field-effect transistors, optoelectronic devices, and gas sensors due to the unique optical properties, sufficiently high carrier mobility, and excellent chemical and thermal stability. In addition, MoS2 has been used as industrial catalysts for many years. For example, the Co-MoS2/Al2O3 catalyst has been industrially used as a hydrodesulfurization catalyst that hydrogenates sulfur contained in crude oil to improve the purity. Thus, the MoS2 surface is a reaction field for various catalytic reactions involving hydrogen atoms. The dissociative adsorption of hydrogen molecules on MoS2 surfaces is considered to be a thermally activated process. At room temperature, the basal plane of MoS2 is inert for the dissociation of molecular hydrogen. Therefore, creating active sites for the hydrogen dissociation on the MoS2 basal surface is expected to lead to higher efficiency of hydrogenation reactions and hydrogen gas sensors using the MoS2 crystal.
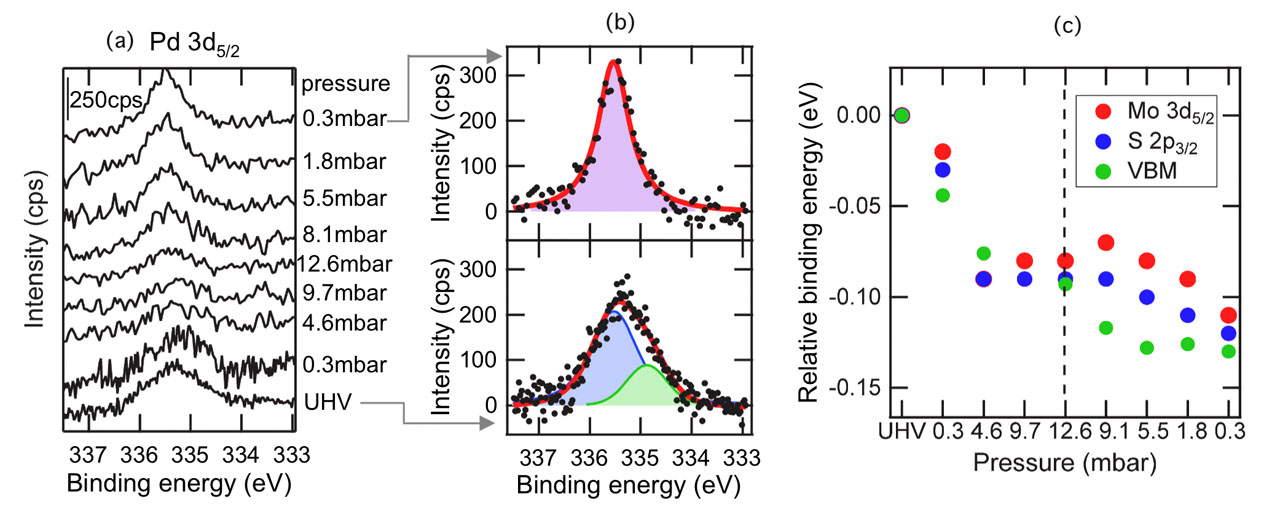
Fig. 1. (a) Pd 3d5/2 spectra of the Pd/MoS2 as a function of H2 gas pressure at 300 K. The amount of deposited Pd was estimated to be 2.0 % for the amount of Mo atoms on the outermost MoS2 layer. (b) The upper and lower figure are the fitting results for the Pd 3d5/2 AP-XPS spectra before and after the hydrogen exposure, respectively. The green component and blue component in the lower figure are assigned to be surface and bulk components of the Pd islands, while the pink component would consist of both the bulk component and hydrogen atoms-adsorbed surface component. (c) The relative binding energies for the Mo 3d5/2 core-level, S 2p3/2 core-level, and valence band maximum (VBM) as a function of H2 gas pressure.
We investigated the effects of gaseous hydrogen exposure on the bare MoS2 and Pd-deposited MoS2 basal surfaces using ambient-pressure X-ray photoelectron spectroscopy [1]. In the Mo 3d core-level, S 2p core-level, and valence-band photoelectron spectra of a bare MoS2 surface, little change was observed in the energy shift before and after exposure to hydrogen gas. Upon hydrogen gas exposure on the Pd-deposited MoS2 surface, the Pd 3d XPS spectra changed (Figs. 1(a) and (b)), which can be interpreted as the adsorption of dissociated hydrogen atoms on the Pd sites; each peak in the photoelectron spectra (Mo 3d, S 2p, and valence-band) shifted to a lower binding energy with 0.1 eV (Fig. 1(c)). When hydrogen gas was evacuated, the peak energy remained lower compared with that before hydrogen gas exposure (Fig. 1(a)). These results indicate that the dissociation of molecular hydrogen and the adsorption of atomic hydrogen occur on the Pd-deposited sites on the MoS2 surface, and thereafter hydrogen atoms spillover onto the MoS2 surface. This study shows that the active site for the dissociation of molecular hydrogen is created on an inert MoS2 basal surface by Pd deposition. In addition, the electronic states of the MoS2 substrate have been modulated by hydrogen atoms spilled over onto the MoS2 surface. These processes and the energy diagram are schematically shown in Fig. 2.
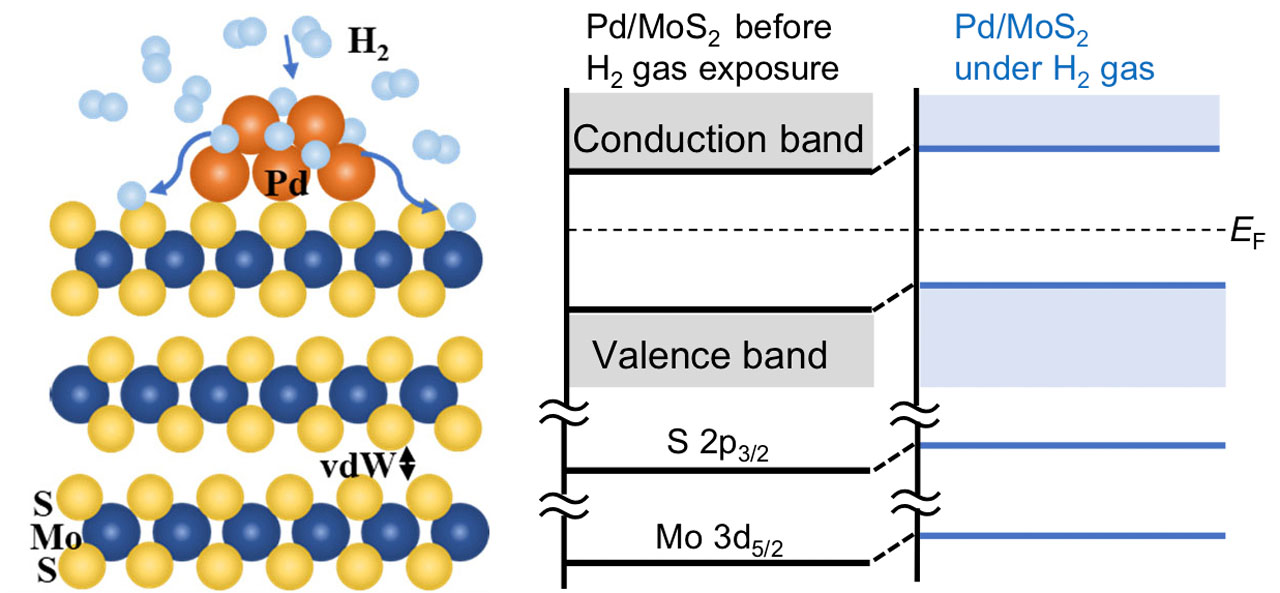
Fig. 2. (Left) The schematic of the dissociation of molecular hydrogen, the adsorption of atomic hydrogen on the Pd-deposited sites on the MoS2 surface and the spillover of hydrogen atoms onto the MoS2 surface. (Right) The energy diagram of the Pd-deposited MoS2 surface before and after hydrogen exposure.
References
- [1] F. Ozaki, S. Tanaka, W. Osada, K. Mukai, M.Horio, T. Koitaya, S. Yamamoto, I. Matsuda, and J. Yoshinobu, Applied Surface Science 593, 153313 (2022).
