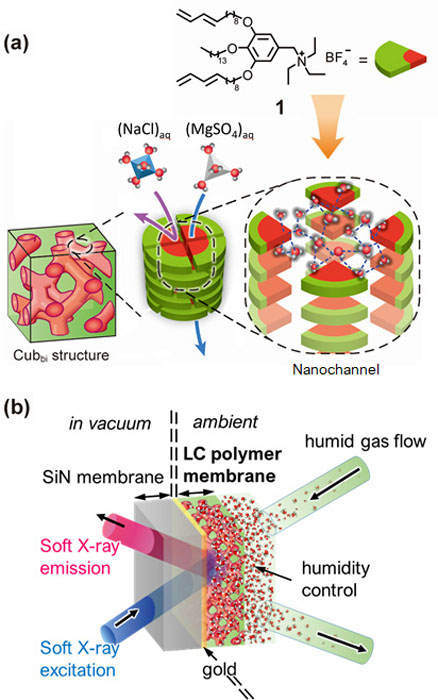
Hydrogen-Bonded Structure of Water Responsible for Ion Selective Permeability of Self-Assembled Liquid Crystalline Polymer Membranes
T. Kato and Y. Harada
Liquid crystals (LC) are appealing for nanofiltration membranes with uniform subnanopores which enable efficient and thermally stable filtration and ion selectivity [1]. Many principles of water filtration are intuitive: particles bigger than the pore size cannot pass through a membrane, and electrostatic interactions between ions, water molecules and the membrane can aid the process. However, the permeability of these LC membranes is higher for larger, dianionic SO42− ions than it is for monoanionic, smaller Cl− ions, which could not be explained using the common filtration principles, which implies the presence of another driving force for ion selectivity. One of such possibility is the structure of the water surrounding the ions
Here we investigated the hydrogen-bonded configuration of water molecules in the subnanopores of a bicontinuous cubic LC membrane formed by in-situ polymerization of compound 1 (Fig. 1a), and how it affects the permeation of dianionic SO42− and monoanionic Cl−[2]. We used high-resolution X-ray emission spectroscopy (XES) [3] to learn how water arranges itself in the subnanopores.
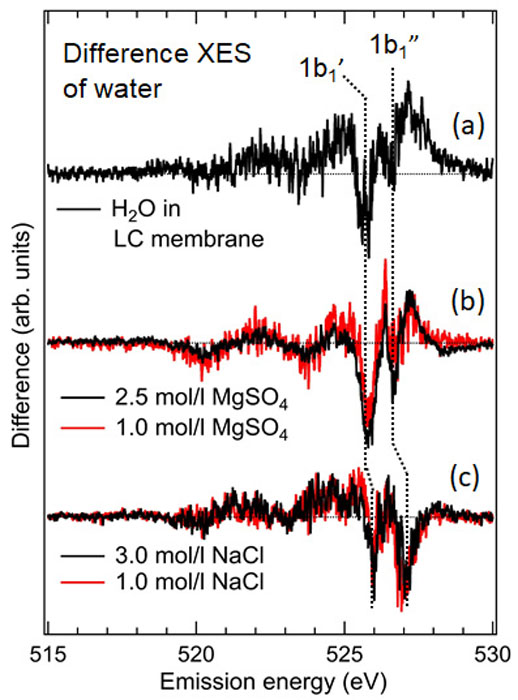
XES spectra of pure liquid H2O absorbed in the LC membrane and hydrating water of permeating solutes, MgSO4 and NaCl were collected at the SPring-8 BL07LSU HORNET station using a high-resolution XES spectrometer [4]. A custom-made ambient pressure cell was used to expose the LC membrane to humidity-controlled moisture and nitrogen as a carrier gas. (Fig. 1b). To explore the origin of the selective ion permeation (73±5% for MgSO4 and 30±6% for NaCl), hydrogen-bonded structures of H2O in the LC membrane were compared with those in the MgSO4 and NaCl hydrations. Spectra of water within the subnanopores (Fig. 2a) revealed that its hydrogen-bonded network, when confined, loses some structural features characteristic of bulk water in favor of new structural features. Very similar structural features are observed in the spectra of the hydration shell of SO42− (Fig. 2b) but not in that of Cl− (Fig. 2c). We interpret this as the reason for the greater membrane permeability of SO42− than that of Cl−. In the near future computational studies might help understand the exact structure of the hydrogen-bonded network within the subnanopores. The nature of hydrogen-bonded networks becomes an additional parameter that should be considered and controlled to obtain highly efficient water filtrations.
References
- [1] (a) T. Kato et al., Nat. Rev. Mater. 2, 17001 (2017); (b) T. Ichikawa et al., J. Am. Chem. Soc. 134, 2634 (2012). (c) M. Henmi et al., Adv. Mater. 24, 2238 (2012). (d) T. Sakamoto et al., Adv. Sci. 5, 1700405 (2018). (e) K. Hamaguchi et al., ACS Macro Lett. 8, 24 (2019). (f) M. Gupta et al., ACS Macro Lett. 8, 1303 (2019).
- [2] R. Watanabe, T Sakamoto, K. Yamazoe, J. Miyawaki, T. Kato, and Y. Harada, Angew. Chem. Int. Ed. 59, 23461 (2020).
- [3] T. Fransson et al., Chem. Rev. 116, 7551 (2016).
- [4] Y. Harada et al., Rev. Sci. Instrum. 83, 013116 (2012).