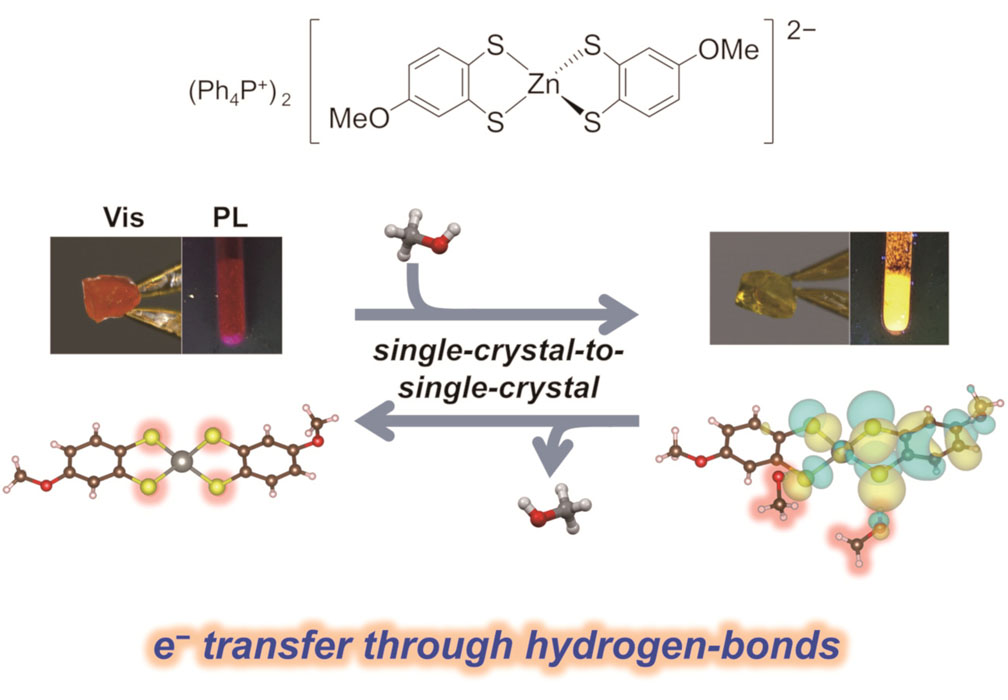
Vapochromism Induced by Intermolecular Electron Transfer Coupled with Hydrogen-Bond Formation in Zinc Dithiolene Complex
Mori and Ozaki Groups
Reversible control of electronic functionalities of molecular materials using external stimuli and perturbations is important not only for applications to next-generation switching devices and sensors but also from the viewpoint of basic science to elucidate the mechanism and to establish the material design criteria. Recently we have demonstrated a proton-electron coupled phenomenon in H (hydrogen)-bonded π-conjugated molecular crystals [1-3], in which magnetism and electrical conductivity were switched by proton (deuteron) dynamics in an intermolecular H-bond. If control of the functionalities, such as optical properties, other than magnetism and electrical conductivity by modulating the electronic states through H-bonding is well established, it will impact various research fields and greatly expand the design criteria of functional materials. As one of the functional molecular materials with optical properties responsive to external stimuli, vapochromic materials, which respond with color changes when exposed to solvent vapors, have been actively studied as good candidates for their use as chemical sensors because they can directly visualize external environmental changes. In this study, we focused on a novel Zn complex, (Ph4P)2[Zn(4-mxbdt)2] (1 in Fig. 1; 4-mxbdt = 4-methoxybenzene-1,2-dithiolate) to realize the control of vapochromic behavior by direct modulation of electronic states through H-bonding. Based on the detailed single-crystal structure analyses and first-principles calculations considering intermolecular interactions using the periodic structure model of the crystal, we disclosed a new mechanism of vapochromism in 1, that is, not molecular structure and arrangement changes but electron transfer via H-bond formation for the first time [2].
The Zn complex 1 was synthesized by complexation of zinc(II) chloride and the ligand precursor in methanol. Yellow block-like crystals were obtained by the slow evaporation of the crude mixture. From the X-ray structural analysis, the yellow crystal was determined as (Ph4P)2[Zn(4-mxbdt)2]·2CH3OH (= 1·2MeOH) (Fig. 1). In this crystal, the [Zn(4-mxbdt)2] complex showed tetrahedral coordination without a π-stacking structure because of the presence of bulky Ph4P+ cations, and thus there were no significant intermolecular interactions between the complexes. The spaces in the loosely packed structure were filled with two methanol molecules per formula. These methanol molecules formed [S···H–O] H-bonds with S atoms on the [Zn(4-mxbdt)2]2-. Interestingly, drying the crystals overnight under vacuum induced the color change from yellow to orange, maintaining its high crystallinity (Fig. 1).
We quantitatively evaluated the color change of each crystal by UV-vis absorption spectra. Visible-light absorption edges of the orange crystal 1 and yellow crystals, 1·H2O and 1·2MeOH, were estimated to be 2.29 eV (541 nm), 2.41 eV (514 nm), and 2.45 eV (506 nm), respectively. A blue shift of the absorption edge (0.12–0.16 eV) upon methanol or water vapor absorption was observed, which is consistent with the visually observed color changes. We also evaluated the photoluminescence properties of these crystals. Significantly, a blue shift was observed in the photoluminescence spectra (0.10–0.14 eV) for both 1·H2O and 1·2MeOH, similar to the UV-vis absorption spectra. The apparent color changed from red to yellowish-orange. These experimental data demonstrate that crystal 1 is a novel vapochromic material having both visible-light absorption and photoluminescence changes when exposed to methanol or water sorption with reversible SCSC (single crystal-single crystal) transformations. Remarkably, this is the first report of metal-dithiolene-based vapochromic materials with SCSC transformations.
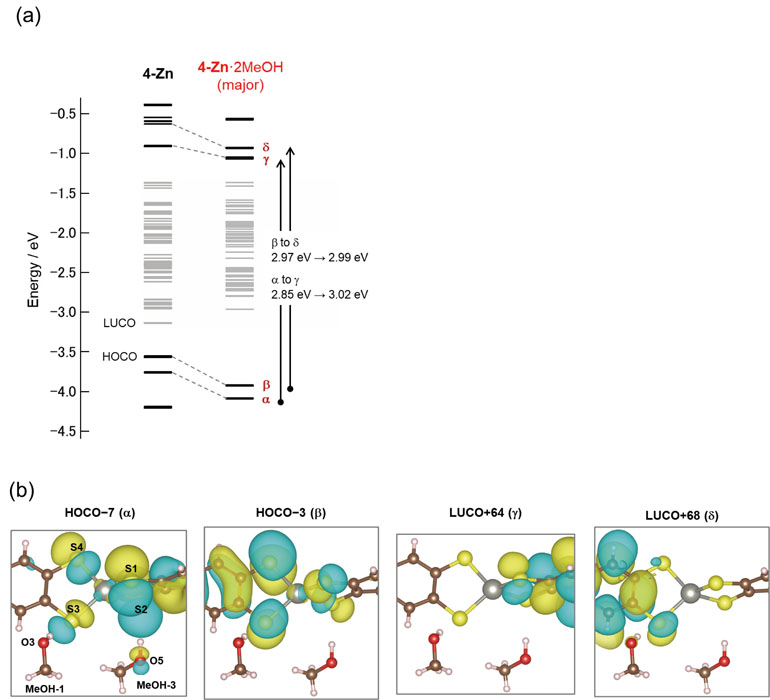
Then, we focused on the mechanism of vapochromism in this crystal, 1·2MeOH. DFT calculations based on the experimentally observed crystal structures using OpenMX software revealed the mechanism of the Fig. 2(b) H-bonding structures in 1·2MeOH. In 1·2MeOH (major), energy gaps for both α to γ and β to δ transitions increased (α to γ: 2.85 eV to 3.02 eV, β to δ: 2.97 eV to 2.99 eV), where the α and β orbitals were more stabilized than γ and δ (Fig. 2 (a)). This is because of the fact that the occupied orbital groups (α and β) have a large weight on the H-bonding S atoms, on which the largest changes in the number of electrons were estimated by Mulliken population analysis, compared with the unoccupied orbital groups (γ and δ). Considering the orbital shape around the H-bond, these crystal orbitals are derived from the hybridization of the frontier orbitals (HOMO-1, HOMO, LUMO, and LUMO+1) of the [Zn(4-mxbdt)2]2- complex with the LUMO of the absorbed vapor molecules at higher energies. This hybridization leads to the above-mentioned electron transfer to stabilize both the α/β and γ/δ orbitals.
In conclusion, a novel mechanism of vapochromism based on the intermolecular electron transfer coupled with H-bond formation was realized in the newly obtained zinc dithiolene complex crystal, (Ph4P)2[Zn(4-mxbdt)2]2CH3OH. According to the mechanism proposed in this study, molecules in which molecular orbitals for visible-light absorption are significantly distributed to H-bonding parts can be promising candidates for a new class of vapochromic materials. The findings here provide important insights to expand the scope of molecular designs for vapochromic as well as other stimuli-responsive materials.
References
- [1] S. Yokomori, A. Ueda, T. Higashino, R. Kumai, Y. Murakami, and H. Mori, Cryst Eng Commun. 21, 2940 (2019).
- [2] S. Yokomori, S. Dekura, T. Fujino, M. Kawamura, T. Ozaki, and H. Mori, J. Mater. Chem. C. 8, 14939 (2020).
- [3] S. Yokomori, S. Dekura, A. Ueda, R. Kumai, Y. Murakami, and H. Mori, J. Mater. Chem. (to be published).