Sandwich Structure Formation of BN-Covered Silicene on ZrB2/Si(111) by Adsorption of NO and Thermal Processes
Yoshinobu Group
Atomic layer materials have attracted much attention in physics, chemistry and materials science. Silicene is a silicon analogue of graphene. A honeycomb silicene layer has been prepared on suitable substrates such as Ag, ZrB2, Ir, ZrC surfaces. It has been reported that a silicene layer grown on these metallic surfaces has relatively strong interaction with the substrate because of the orbital mixing between metal bands and Si sp-bands. Towards the functionalization of silicene for device applications, several theoretical and experimental investigations have been carried out concerning the interaction between the silicene and other atoms/molecules. Here, we investigated the adsorption and thermal reaction processes of NO with the a silicene monolayer on the ZrB2/Si(111) substrate using synchrotron radiation X-ray photoelectron spectroscopy (SR-XPS) and density-functional theory (DFT) calculations. SR-XPS experiments were carried out at BL13 of KEK-PF.
Figure 1 shows a series of O 1s, N 1s, Si 2p and B 1s XPS spectra of these processes. The initial surface is a clean silicene monolayer on ZrB2/Si(111) (see each XPS for the clean surface). NO is dissociatively adsorbed on the silicene surface at 300 K. At an early stage of NO exposure, an atomic nitrogen is bonded to three Si atoms most probably by a substitutional adsorption with a Si atom of silicene (N≡Si3). An atomic oxygen is inserted between two Si atoms of the silicene (Si-O-Si). After a large amount of NO exposure (1500 L), the two-dimensional honeycomb silicene structure becomes destroyed judging from the decay of typical Si 2p spectra for silicene (see Fig. 1c). The oxidation state of Si becomes Si4+ predominantly and the XPS peaks of ZrB2 substrate are decreased in intensity, indicating that complicated silicon oxinitride species have developed three-dimensionally on the ZrB2/Si(111) substrate at 300 K. By heating above 900 K, the oxide species start to desorb from the surface probably as SiO, but nitrogen-bonded species still exist (Figs. 1a and 1b). After heating at 1053 K for 5 min., no oxygen species is observed on the surface (Fig. 1a); SiN species are temporally formed as a metastable species and BN species also start to develop (Fig.1b). In addition, the silicene structure is partly restored on the ZrB2/Si(111) substrate (Fig. 1c).
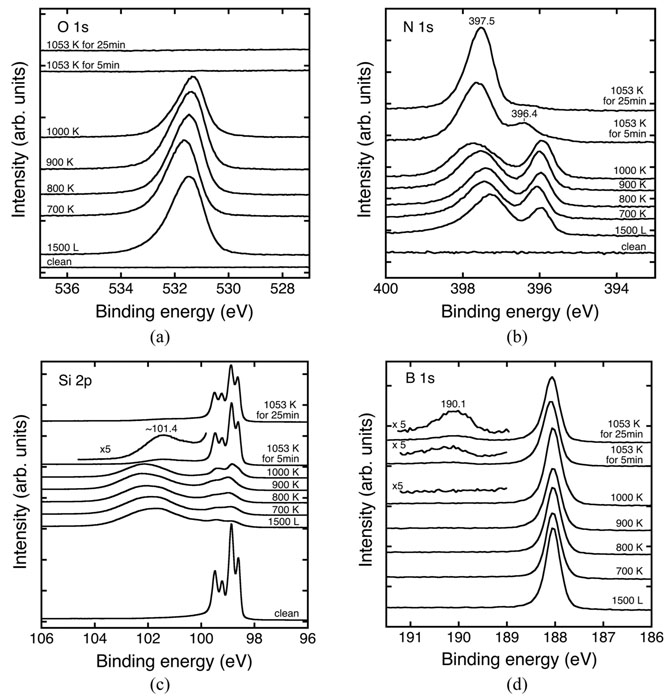
Fig. 1. A series of XPS spectra of the 1500 L NO/silicene/ZrB2/Si(111) surface as a function of heating temperature. (a) O 1s spectra (hn = 700 eV), (b) N 1s spectra (hn = 560 eV), (c) Si 2p spectra (hn = 260 eV), and (d) B 1s spectra (hn = 700 eV). After quick heating to each temperature, the sample was cooled down to room temperature, and XPS measurements were carried out. Note that at 1053 K, the heating duration time was 5 min and 25 min.
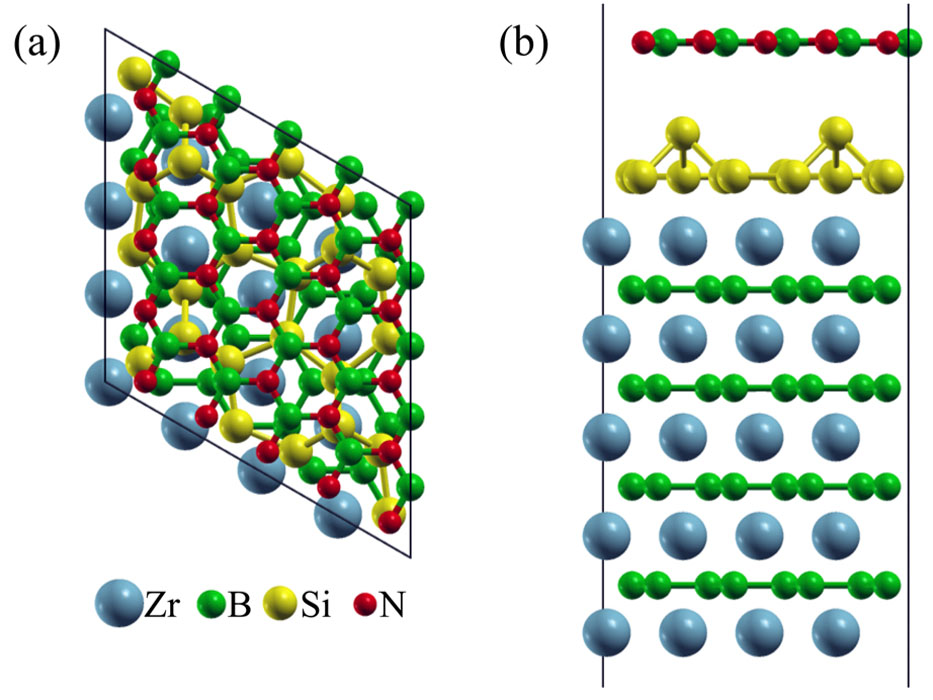
After the prolonged annealing at 1053 K for 25 min., the XPS spectra show a single peak at 397.5 eV for N 1s (Fig. 1b) and an emerging peak at 190.1 eV for B 1s state (Fig. 1d). Since the B 1s peak at 190.1 eV can be ascribed to the B atom in the hexagonal BN structure according to the reference XPS spectra of BN, we conclude that the hexagonal BN structure has developed on the silicene on ZrB2. By DFT calculations, the geometrical structure has been optimized as shown in Fig. 2; a hexagonal BN sheet weakly interacts with the silicene layer on the ZrB2 substrate. The calculated binding energy of the N 1s state in the hexagonal BN is 397. 61 eV on average, while there is a variation of the binding energies depending on the relative position of N atoms with respect to the Si atoms of silicene, and the minimum and maximum binding energies are 397.41 and 397.81 eV, respectively. The good agreement between the experimental and calculated binding energies supports the formation of the hexagonal BN structure after the prolonged annealing at 1053 K. Thus, the sandwich structure of BN-covered silicene on the ZrB2/Si(111) substrate is formed by adsorption of NO at 300 K and prolonged heating at 1053 K.
References
- [1] J. Yoshinobu et al., J. Chem. Phys. 153, 064702 (2020). doi: 10.1063/5.0011175