Operando Soft X-ray Emission Spectrosopy of Fe2O3 Anode for Li-Ion Battery
D. Asakura, E. Hosono, J. Miyawaki, and Y. Harada
Improving the energy and power density of electrode materials for Li-ion batteries (LIBs) is highly important to further develop electric vehicles. For the improvements of battery performance, understanding the charge (Li-extraction) and discharge (Li-insertion) mechanisms from the viewpoint of the electronic structure is indispensable. Here, we demonstrate operando Fe L3-edge resonant XES for Fe2O3 which is an anode material of LIB [1]. It is known that the discharge/charge mechanism is based on conversion reaction denoted as Fe2O3 + 6Li+ + 6e- ⇔ 3Li2O + 2Fe [2,3]. To confirm the conversion reaction and further investigate the electronic-structure charge, we analyzed the operando XES spectra using full-multiplet calculation [4,5].
The operando cell consists of Fe2O3 poly-crystalline thin film (~100 nm), a Li-metal counter electrode, and an electrolyte solution [1,6]. The operando XES experiments were carried out with the HORNET XES spectrometer at BL07LSU of SPring-8 [7]. The electrochemical discharge/charge was performed by cyclic voltammetry. The operando XES measurements were at an open-circuit voltage (OCV), discharged state and charged state on the second discharge-charge cycle.
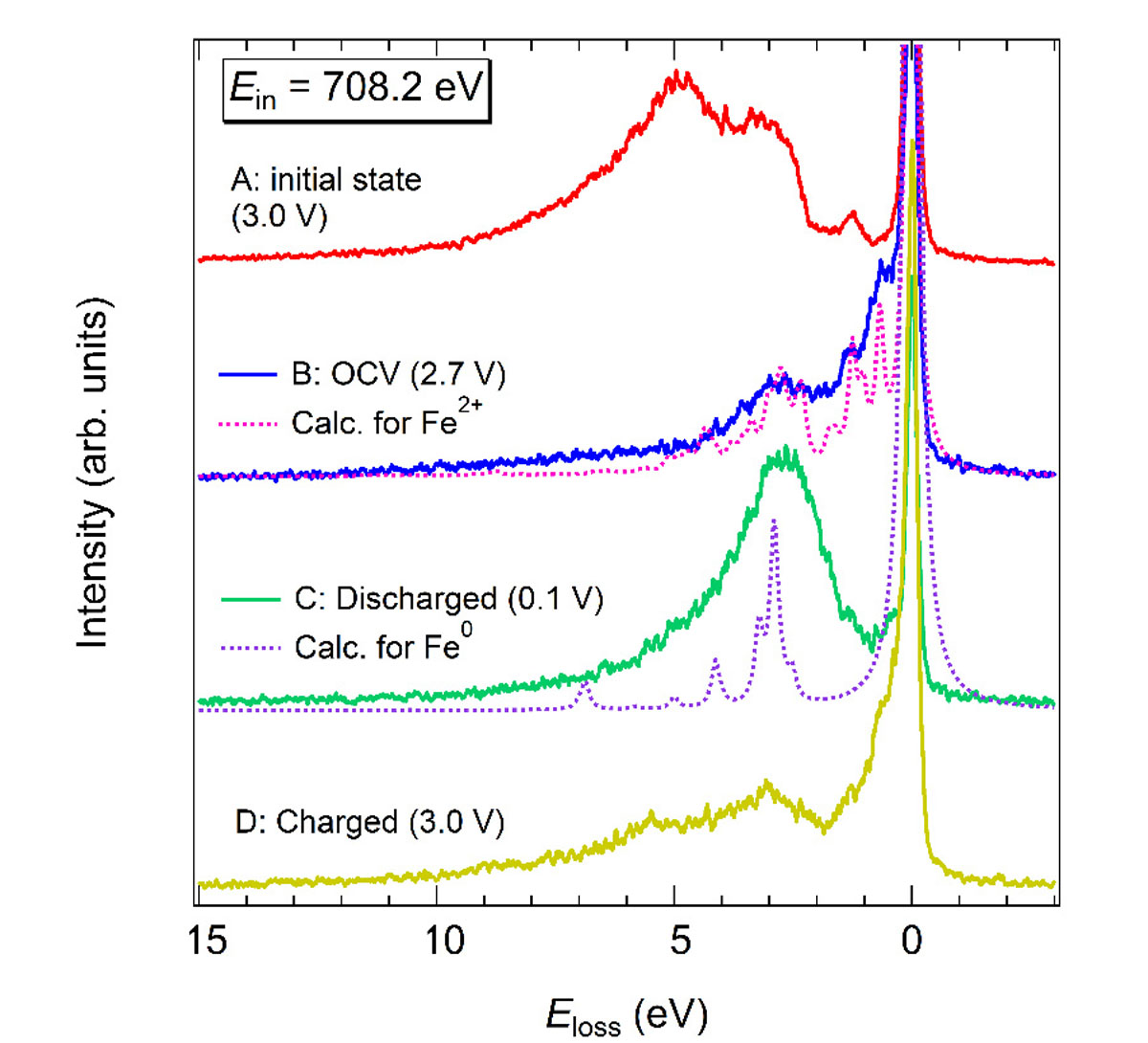
Figure 1 shows the XES results [1]. The XES spectrum for the initial state is attributed to Fe3+ state because the XES line shape is almost the same as that for Fe2O3 in previous reports [8]. On the other hand, the valence of Fe at the OCV before the second cycle was not Fe3+, but Fe2+ with a weak p-d hybridization, suggesting considerable irreversibility on the first discharge-charge cycle and a weakened Fe-O bond after the first cycle. Moreover, we revealed that the Fe 3d electronic-structure change during the second cycle was to some extent reversible as Fe2+ (2.7 V vs. Li/Li+: OCV) → Fe0 (0.1 V vs. Li/Li+: discharged) → Fe(2+d)+ (3.0 V vs. Li/Li+: charged). This operando Fe L3-edge resonant XES in combination with the full-multiplet calculation provides detailed information for redox chemistry during a discharge-charge operation that cannot be obtained by other methods such as crystal-structure and morphology analyses. XES is thus very powerful to investigate the origin and limitation of the lithiation function of anodes involving conversion reaction.
References
- [1] D. Asakura et al., Phys. Chem. Chem. Phys. 21, 26351 (2019).
- [2] S. Morzilli et al., Electrochem. Acta 30, 1271 (1985).
- [3] J. Chen et al., Adv. Mater. 17, 582 (2005).
- [4] D. Asakura et al., J. Phys. Chem. Lett 5, 4008 (2014).
- [5] D. Asakura et al., Phys. Chem. Chem. Phys. 21, 18363 (2019).
- [6] D. Asakura et al., Electrochem. Commun. 50, 93 (2015).
- [7] Y. Harada et al., Rev. Sci. Instrum. 83, 013116 (2012).
- [8] J. Miyawaki et al., Phys. Rev. B 96, 214420 (2017).