Effects of Molecular Dynamics on the Anhydrous Proton Conductivity of Imidazolium Hydrogen Dicarboxylates
Mori Group
Hydrogen (H) is a ubiquitous element, which plays important role in a wide range of fields from the deep underground to the interstellar space, and from inorganic oxides to biosystems. Proton (H+) migration in solids is one of such extensively studied research topics. Recently, anhydrous organic proton conductors, which can show proton conductivity at above 100 °C without humidification, have attracted scientific interests owing not only to their wide applicability as solid-state electrolytes for fuel cells, but also to their peculiar proton conduction mechanism mediated not by water molecules. However, there have been only a few reports about anhydrous organic proton conductors.
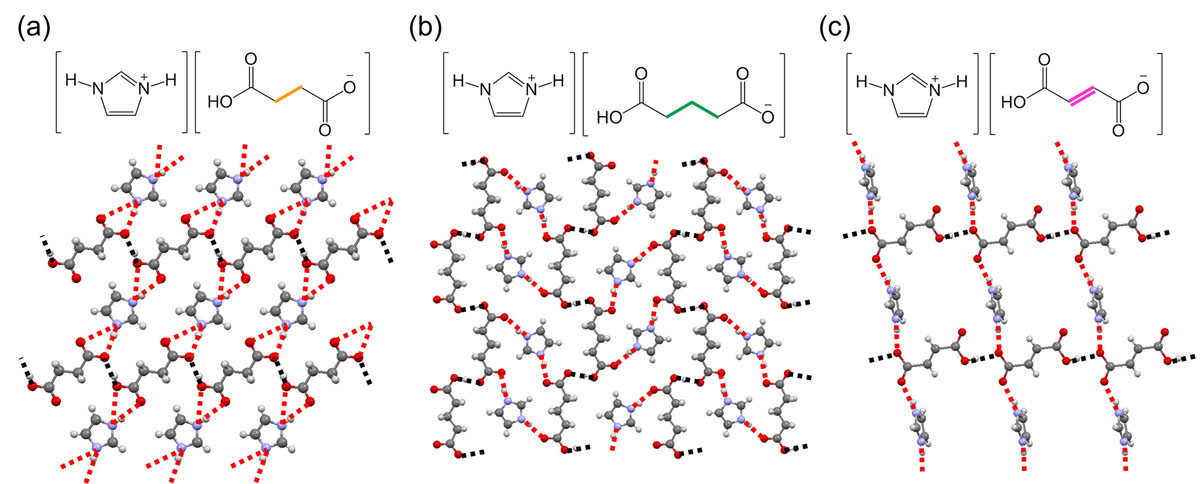
Previously, we investigated the “intrinsic” proton conductivities of imidazolium succinate (= Im-Suc; Fig. 1a) and glutarate (= Im-Glu; Fig. 1b), by single-crystal measurements, and successfully disclosed two key factors for realization of anhydrous proton conductivity: (1) the hydrogen-bond (H-bond) network structures, and (2) difference of the proton donating/accepting abilities between acid and base molecules, i.e., ΔpKa [1]. In this study, we newly prepared high-quality single crystals of another salt, imidazolium fumarate (Im-Fum; Fig. 1c), in addition to Im-Suc and Im-Glu, and investigated the effect of molecular “dynamics” on the anhydrous proton conductivities of these three salts [2].
Im-Fum has similar 2D H-bond network structure as Im-Suc and Im-Glu (Fig. 1), and showed the anisotropic proton conductivity consistent with the previously disclosed “static” key factors (1) and (2). Interestingly, Im-Fum exhibits linear dependence on the reciprocal temperature in contrast to non-linear dependence in Im-Suc and Im-Glu. We speculated that it is contributed by the imidazolium cation in the 2D H-bond network because the Ea showed quite high values in Im-Suc and Im-Glu similar to the other imidazolium-based salts, and that the molecular orientation of imidazolium cation is different between Im-Fum and the other two. Thus, we investigated the dynamic property of imidazolium cations in these salts by high-temperature X-ray structure analyses and solid-state 2H NMR.
In Im-Suc, a structural transition was observed in X-ray structural analysis at ca. 80 °C, accompanied with the in-plane orientational disorder of imidazolium molecules in one out of two layers. Consistently, the proton conductivity showed abrupt increase at ca. 80 °C, which indicates that the imidazolium libration motion promotes proton conduction in Im-Suc (Fig. 2 upper). On the other hand, no significant changes were observed for Im-Glu and Im-Fum in X-ray analyses.
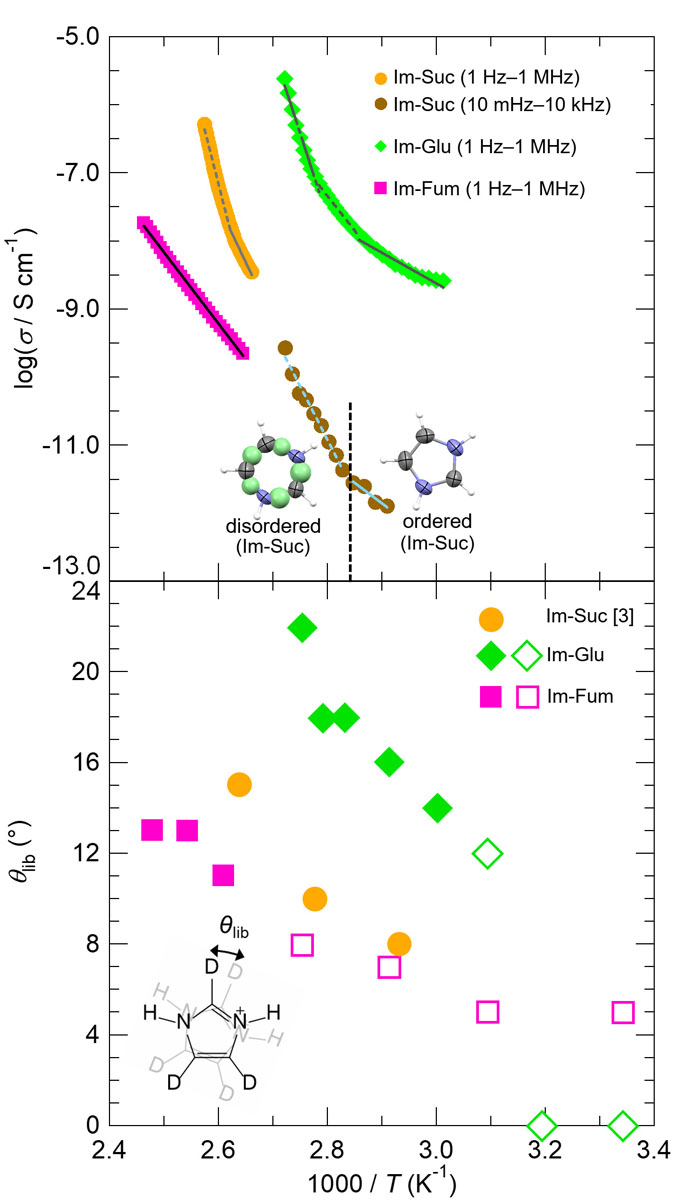
Fig. 2. (upper) The single-crystal proton conductivity (σ) and (lower) libration angle θlib vs. 1000/T plots for Im-Suc (orange and dark orange circles), Im-Glu (green diamonds), and Im-Fum (pink squares). In upper graph, the lines denote the Arrhenius fitting, and the inset figures show ordered/disordered imidazolium cation in Im-Suc below/above the transition temperature, ca. 80 °C. In bottom graph, filled circles, diamonds, and squares denote libration angles of Im-Suc (orange)[3], Im-Glu (green), and Im-Fum (pink) in the proton-conducting temperatures, and open markers denote those below proton-conducting temperatures. Inset shows the model for the libration motion of imidazolium cations.
Then, in order to investigate the local dynamics of imidazolium cations, we performed the solid-state 2H NMR experiments for Im-Glu and Im-Fum using imidazole-d3 molecules (Fig. 2 lower inset). By spectral simulations assuming the two-site jump model describing the libration motion of imidazolium molecules, we estimated the librational rates klib and angles θlib. Comparing the libration angles θlib and proton conductivities σ of Im-Glu and Im-Fum together with the reported values of Im-Suc [3], magnitude of θlib is Im-Glu > Im-Suc > Im-Fum, in consistent with the same relationship of σ. This result clearly indicates that the libration motion of the imidazolium cations promotes proton conduction in these salts.
We consider that this “dynamic” factor synergistically contributes to anhydrous proton conductivity with the already mentioned the “static” factors. Imidazolium molecules lie within the 2D H-bond network in Im-Suc and Im-Glu whereas imidazolium sticks out from the network in Im-Fum (Fig. 1). In former case, directions of libration and proton conduction matches, and the libration can make additional H-bonds or short contacts to promote the proton transfer in the H-bond network, leading to the nonlinear dependence of σ in the Arrhenius plot, whereas it is not for the latter case.
In conclusion, in addition to previously disclosed “static” key factors, (1) H-bond network structures and (2) ΔpKa between acid and base molecules, we revealed that (3) the molecular “dynamics” is another key factor for realization of anhydrous proton conductivity. These “static” and “dynamic” factors synergistically play important roles, which is thought as peculiar nature of Grotthuss-type conduction in anhydrous solids.
References
- [1] Y. Sunairi, A. Ueda, J. Yoshida, K. Suzuki, and H. Mori, J. Phys. Chem. C 122, 11623 (2018).
- [2] Y. Sunairi, S. Dekura, A. Ueda, T. Ida, M. Mizuno, and H. Mori, J. Phys. Soc. Jpn. 89, 051008 (2020).
- [3] T. Umiyama, R. Ohashi, T. Ida, and M. Mizuno, Chem. Lett. 42, 1323 (2013).