Geometrical Frustration of B-H Bonds in Layered Hydrogen Borides Accessible by Soft Chemistry
I. Matsuda Group
Electrically conducting materials have been essential elements for devices and also for redox reactions on electrodes to sustain our electronic and information society. Miniaturizations in nanotechnology have demanded to reduce the material size down to the atomic limit, however, there are only a few conducting atomic layers, such as graphene, up to now. A search of a novel atomic layer is significant to produce new physical/chemical properties and also to promote various functionalities by combining different types of layers.
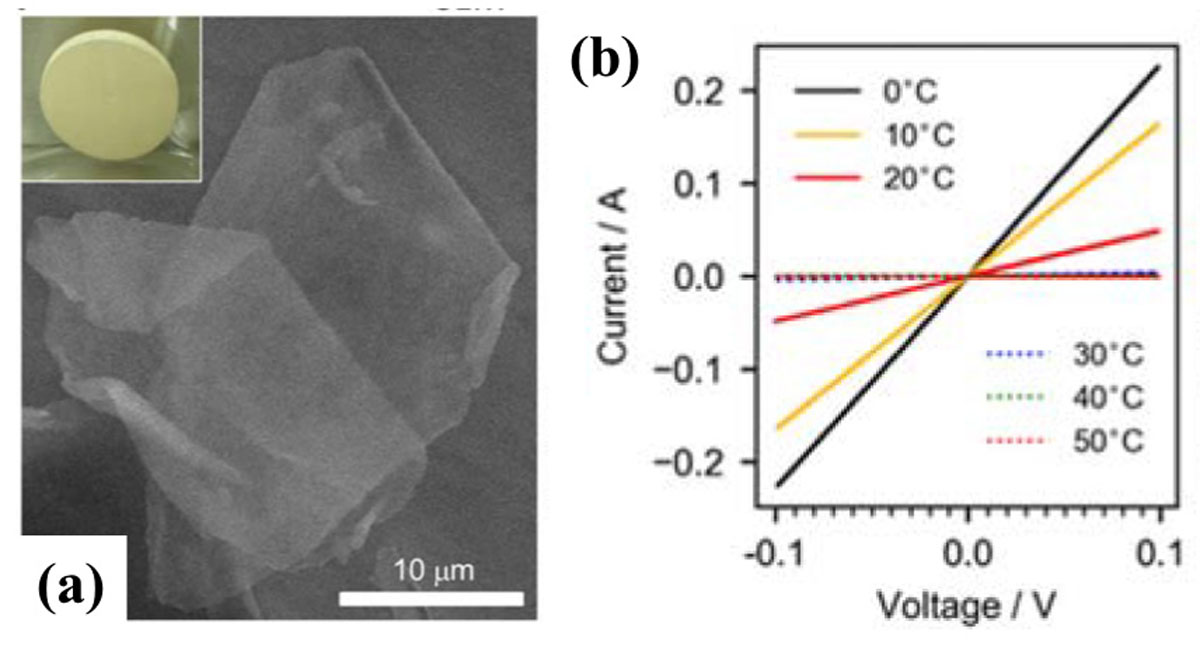
In 2017, atomic sheets of hydrogen boride (HB) or borophene are synthesized by the ion-exchange process [1]. The HB sheet has been theoretically predicted to be conducting. However, preliminary experiments of the transport measurement have resulted in insulating properties. This fact has lead experimentalists to take great efforts of improving the purity of the samples to develop the conductivity. Eventually, in the present research [2], high-purity HB sheets were successfully synthesized, as shown in Fig. 1(a), and achieved the highest conductivity in hydrogen borides (0.13 S/cm). Moreover, suppression of the conductivity in previous samples were found to be due to adsorption of residual organic molecules on the sheet. Interestingly, the HB sheet becomes insulating at 30 °C when there are residual molecules and, reversely, returns to have high conductivity below the temperature, as shown in Fig. 1(b).
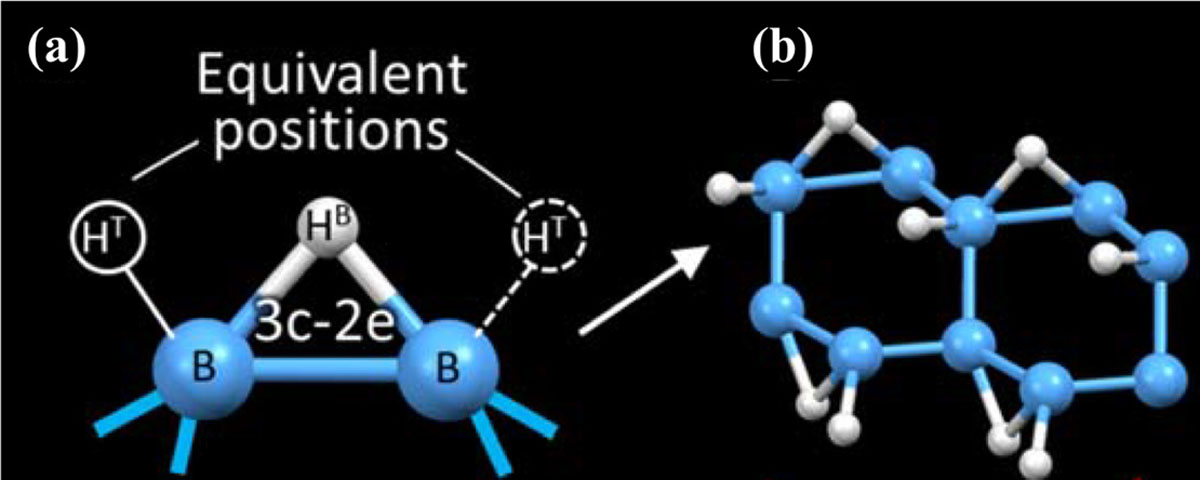
To reveal the intriguing phenomena in terms of the atomic structure, an X-ray scattering experiment was carried out at the synchrotron radiation facility, SPring-8. By analyzing the experimental pair distribution function with the Bayesian optimization, it became clear that there are locally special arrangements of hydrogen atoms in the sheet that allow adsorptions of the residual organic molecules. Figure 2 shows the atomic model. The structure consists of three-center, two-electron (3c-2e) B-H-B bridging bonds as well as ordinary two-center, two-electron (2c-2e) B-H terminal bonds. The present research revealed that conductivity of atomic sheets of hydrogen boride can be regulated by the tiny number of molecules, indicating promising functionalities in sensors or in catalysts.
References
- [1] H. Nishino, T. Fujita, N. T. Cuong, S. Tominaka, M. Miyauchi, S. Iimura, A. Hirata, N. Umezawa, S. Okada, E. Nishibori, A. Fujino, T. Fujimori, S. Ito, J. Nakamura, H. Hosono, and T. Kondo, J Am Chem Soc. 139, 13761 (2017).
- [2] S. Tominaka, R. Ishibiki, A. Fujino, K. Kawakami, K. Ohara, I. Matsuda, H. Hosono, and T. Kondo, Chem 6, 406 (2020).