Highly Homogeneous Gel without Spatial Heterogeneity
Shibayama Group
Fabrication of ordered nanostructure is a crucial step in a wide range of fields, although the typical size of these objects is still limited to a scale of µm3 even with current technologies. Polymer gels are a familiar soft material consisting of a nanoporous three-dimensional network that is readily prepared in a large scale, in principle unlimited. However, application of gels as a nanostructured object is obstructed by the fact that gel networks inevitably have a significant level of defects including dangling ends, loops, entanglements, and nonuniform pore sizes, as a result of the fully stochastic gelation reaction. Numerous attempts have been made to remove these defects from polymer gels, including by synthesizing them from monodisperse polymer chains, by using a relatively uniform cross-linking process such as a photoreaction, by limiting unfavorable intramolecular reactions via the A-B type cross-coupling of star polymers, and even by cross-linking polymer chains with movable cross-linkers. Nevertheless, discernible signs of spatial defects have been persistently observed in gels. Gels have been believed to be inherently dis-ordered as a result of the stochastic reaction.
In this study, we broke this preconception: we present a simple but yet universal scheme to fabricate polymer gels with a highly ordered network. Our strategy is to bring a geometric constraint into the pregel solution so that the space is always uniformly filled with the starting polymer units throughout the gelation reaction (Fig. 1). This gelation framework is known as “bond-percolation” in the classical percolation theory. The spatial and temporal heterogeneity of the synthesized gel network is investigated in the Fourier space by using scattering techniques. Our gel did not show any signatures of heterogeneity: no static laser speckles (Fig. 2), no anomalous small angle scattering, and fully ergodic concentration fluctuations (Fig. 3) and ideal rubber elasticity were observed. These results are completely different from the widely accepted picture of gels. Because both the spatial and temporal correlations of polymer chains in our gels are identical to those of non-crosslinked pre-gel solutions, we cannot even determine the physical state (sol or gel) of our sample by scattering techniques.
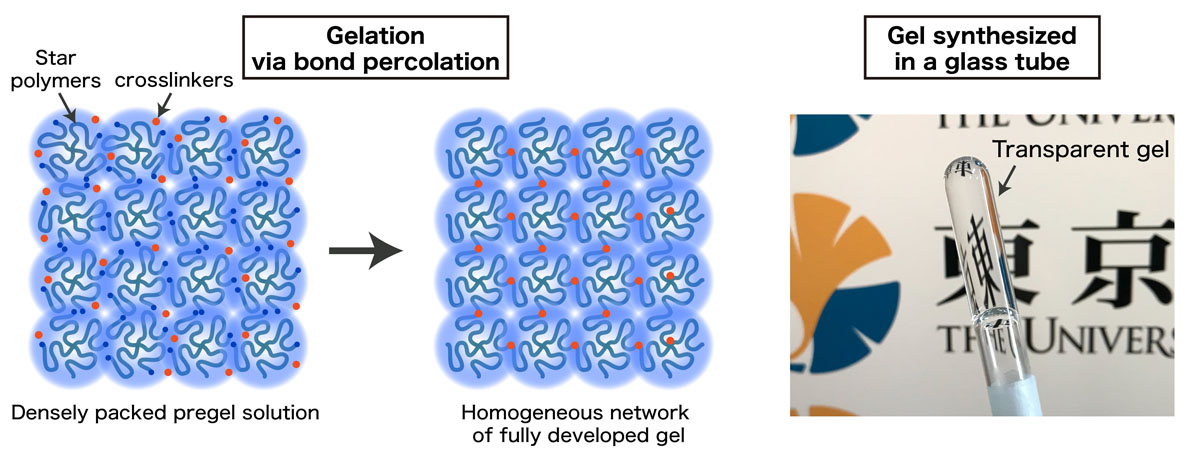
Fig. 1. Illustration of synthesis scheme of highly homogeneous gel via bond percolation. Four-armed star polymer units were dissolved in a good solvent at a concentration much higher than the chain overlapping limit of the polymers. Small crosslinkers were added into the polymer solution. The crosslinkers gradually connect the star polymers and eventually percolate the system. Because there is almost no spatial defect in this polymer gel, the transparency of this material is extremely high.
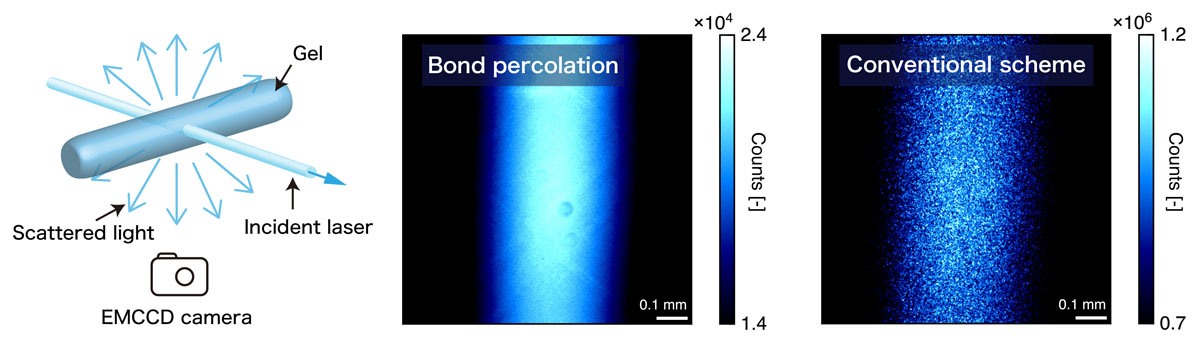
Fig. 2. Static laser speckle test to visualize the spatial defects in the gels synthesized via the bond percolation and conventional scheme. A coherent laser beam was directed to the gel samples and the scattered light was collected using an EMCCD camera. Each image was accumulated for 30 sec to obtain a time-averaged intensity. The bright spots (static laser speckles) in the images denote the static interference from the heterogeneous structures in the gels.
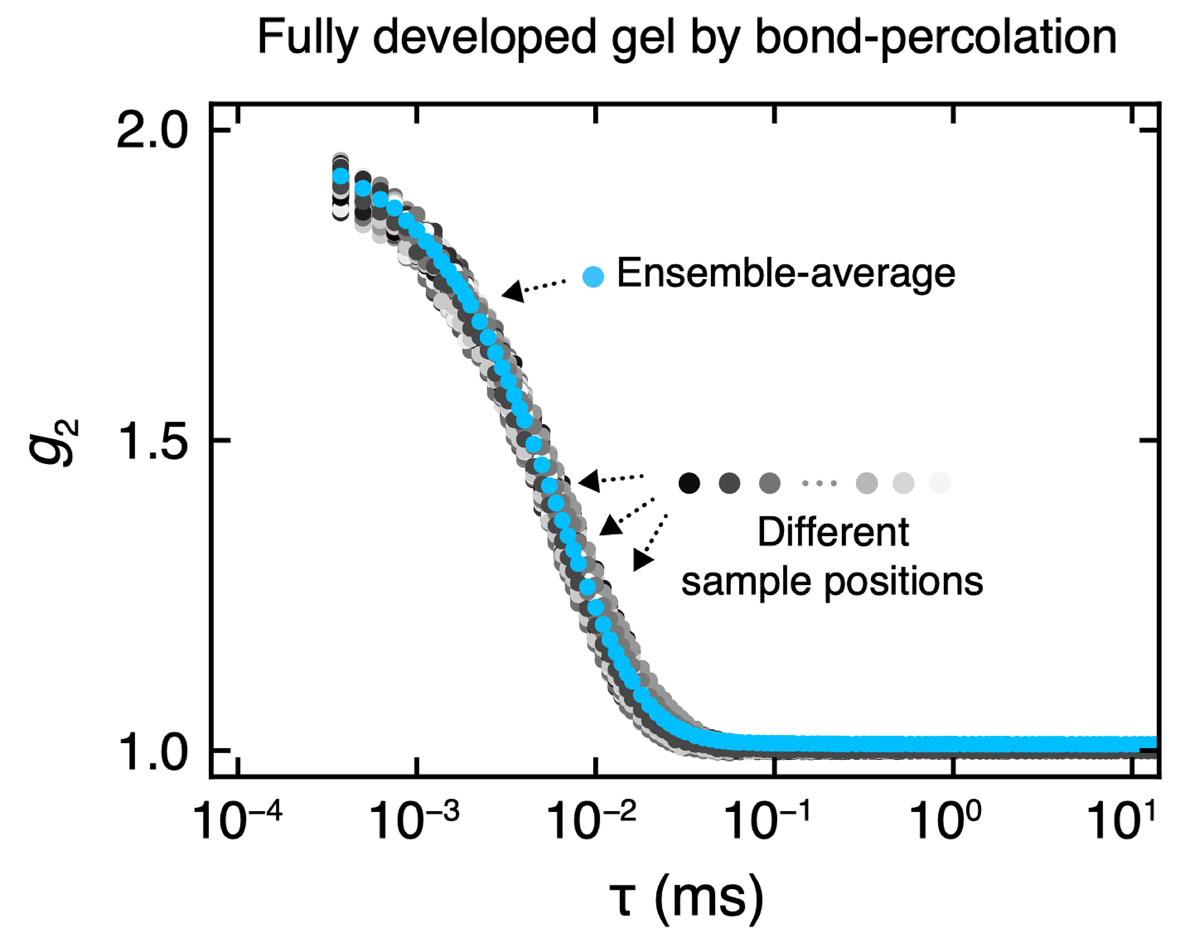
Fig. 3. Intensity time correlation function of the highly homogeneous gel measured with dynamic light scattering at 100 randomly chosen sample positions. All the intensity correlation functions well overlap, indicating the high temporal homogeneity in this gel. The calculated ensemble-averaged intensity correlation function were in perfect consistence with the individual local correlation function, suggesting the ergodic concentration fluctuations of polymer chains in the highly homogeneous gel.
Gels have often been discussed in analogy with glass because both gels and glass have a highly disordered structure. However, our results suggest that the bond percolation gel is more like a crystal than glass because almost identical star polymers are closely packed in the space and no spatial defects were detected. The Bragg peaks of the neighboring polymer units were not observed in this gel because the polymer chains can fluctuate much more than atoms in the crystals. This fluctuation of the polymer chains would lead to substantial blurring of the observable correlation between chains.
The simplicity of our gel preparation scheme will enable us to synthesize homogeneous gels with highly ordered network from a variety of polymers with different chemical structures and functionalities (e.g. polydimethylsiloxane, poly(acrylic acid), and poly(N-isopropylacrylamide)), which will open the door to a wide range of new applications.
References
- [1] X. Li, S. Nakagawa, Y. Tsuji, N. Watanabe, and M. Shibayama, Science Advances. 5, eaax8647 (2019).
