The Roles of Step-Site and Zinc in Surface Chemistry of Formic Acid on Clean and Zn-mModified Cu(111) and Cu(997) Surfaces
Yoshinobu Group
Methanol is of great interest as a source of future hydrogen energy systems, because it is a useful chemical that can produce hydrogen. On the other hand, the synthesis of methanol from CO2 and H2 (CO2 + 3H2 → CH3OH + H2O) is one of the effective use of CO2, where the Cu/ZnO catalyst has been used for this reaction. However, the details of reaction mechanism including intermediate species have not been fully elucidated yet. The chemistry of formic acid (HCOOH) on Cu surfaces is important, because the formate species (HCOO) is one of the stable intermediate species in methanol synthesis.
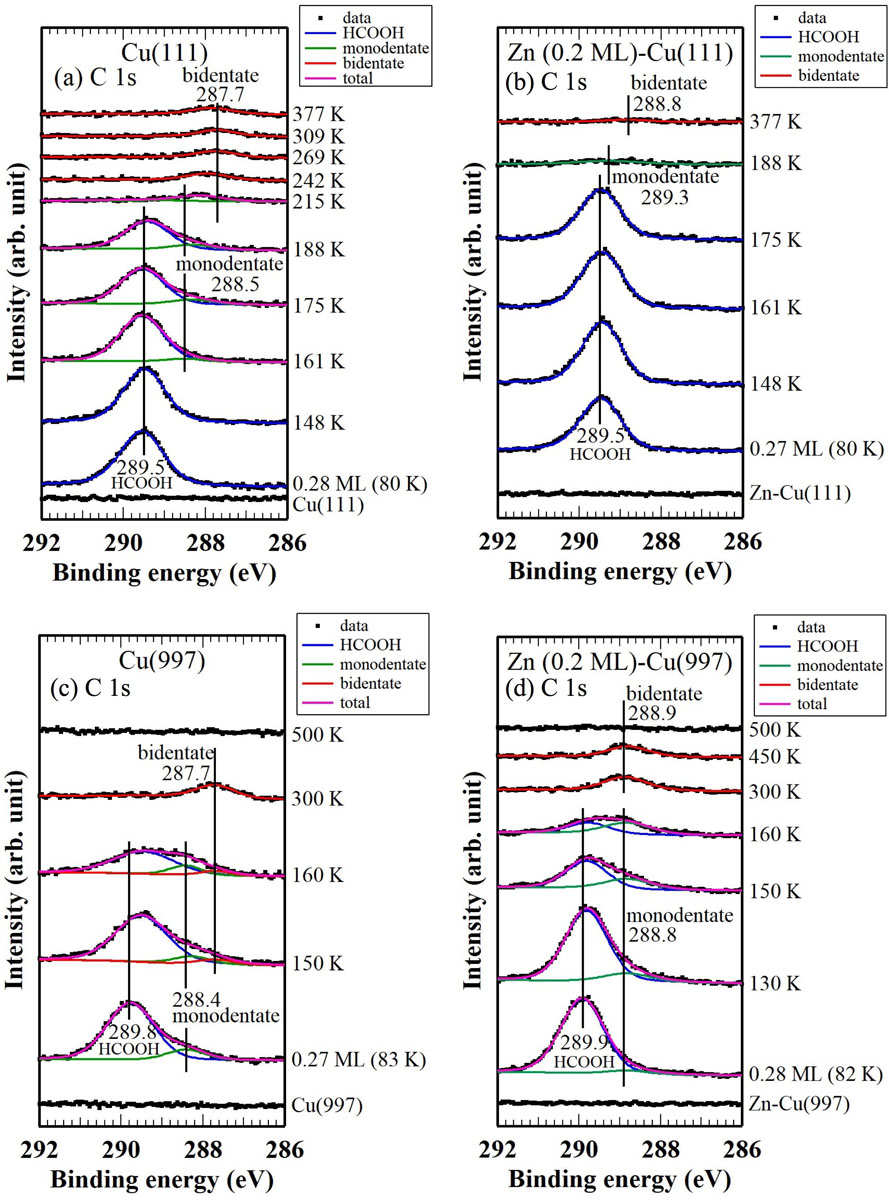
In this study, we systematically investigated the adsorption, desorption and decomposition of formic acid (HCOOH) on Cu(111), Cu(997), Zn-Cu(111) and Zn-Cu(997) using high-resolution X-ray photoelectron spectroscopy (HR-XPS), temperature programmed desorption (TPD) and infrared reflection absorption spectroscopy (IRAS) [1]. On the clean Cu(111) surface, 13% of formic acid molecules adsorbed at 83 K were dissociated to form bidentate formate species by heating at 300 K (Fig. 1a); however, on the Zn-Cu(111) surface, only 4% or less of adsorbed HCOOH were dissociated into the bidentate formate species (Fig. 1b). On the other hand, 13% of adsorbed HCOOH were already dissociated into monodentate formate species on Cu(997) even at 83 K and 17% of adsorbed formic acid molecules were transformed to bidentate formate species by heating (Fig.1c). Thus, the stepped Cu surface has higher reactivity for HCOOH dissociation at low temperature. On the Zn-Cu(997) surface, 20% of formic acid became bidentate formate species in contrast to the case with Zn-Cu(111) (Fig. 1d); the Zn deposited Cu step surface shows special activity for adsorption and dissociation of formic acid.
The desorption peak maxima of the formate decomposition products (CO2 and H2) on Zn-Cu(997) were shifted to higher temperature than those on Cu(997) in TPD results. Thus, the Zn on Cu surfaces plays an important role in the stabilization of formate species, which probably leads to the decrease in the activation barrier for hydrogenation on the Zn-Cu surface. The bidentate formate species at the Zn-Cu sites can remain after heating at 472 K on Zn-Cu(997); we found that the formate species at Zn-Cu step sites are further stabilized. Therefore, in the real Zn-Cu catalysts that have many step sites, we think that the synergy by the Zn and Cu step site can play a vital role in methanol synthesis.
References
- [1] Y. Shiozawa, T. Koitaya, K. Mukai, S. Yoshimoto, and J. Yoshinobu, J. Chem. Phys. 152, 044703 (2020).