Oxygen-Functionalization of Graphene Enhances CO2 Adsorption under Near-Ambient Conditions
S. Yamamoto, J. Yoshinobu, and I. Matsuda
The functionalization of graphene is important in practical applications of graphene, such as in heterogeneous catalysts. The adsorption of molecules on functionalized graphene is an important elementary step in catalytic reactions on graphene. Despite its importance, however, experimental approaches to clarify the interactions of adsorbed molecules with functionalized graphene are limited. Especially, the experiments in ambient conditions at which catalysts are operated have been challenging.
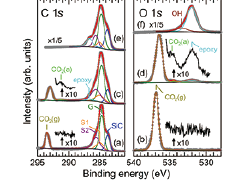
Fig. 1. (a, b) C 1s and O 1s XPS spectra of the pristine epitaxial graphene measured in 1.6 mbar CO2 at 175 K. (c, d) C 1s and O 1s XPS spectra of the oxygen-functionalized epitaxial graphene measured in 1.6 mbar CO2 at 175 K. (e, f) C 1s and O 1s XPS spectra of the oxygen-functionalized epitaxial graphene measured in UHV at 199 K after evacuating the CO2. The incident photon energy was 740 eV. The photon flux densities were 1.0 × 1016 photons/s·cm2 for (a, b), 7.3 × 1016 photons/s·cm2 for (c, d), and 1.5 × 1016 photons/s·cm2 for (e, f). The XPS spectra were normalized with the photon flux densities.
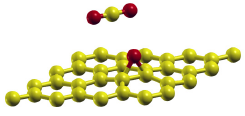
Fig. 2. The structural model of CO2 adsorbed on the oxygen-functionalized graphene. The oxygen species on graphene is an epoxy (C-O-C) group.
In this study [1], the adsorption of CO2 on an oxygen-functionalized epitaxial graphene surface was studied at near-ambient conditions using ambient-pressure X-ray photoelectron spectroscopy (AP-XPS). Monolayer epitaxial graphene on SiC(0001) was used in this study. The oxygen-functionalization of graphene was achieved in-situ by the photo-induced dissociation of CO2 with X-rays on graphene in a CO2 gas atmosphere. AP-XPS experiments were performed at SPring-8 BL07LSU.
Figures 1(a) and (b) shows C 1s and O 1s XPS spectra of the graphene surface measured under 1.6 mbar CO2 at 175 K. Except for the spectral features of the substrate (Graphene(G), SiC, and buffer layer (S1 and S2)) and gas-phase CO2 (CO2(g)), no adsorbed CO2 molecules were observed on graphene under the present condition. When the photon flux density was increased by a factor of ~7 under the same conditions of CO2 gas pressure and sample temperature, C 1s and O 1s XPS spectra were changed (Figs. 1(c, d)). New small peaks at 291.2 eV in C 1s and at 534.7 eV in O 1s XPS spectra were ascribed to adsorbed CO2 [2]. Figures 1(e) and (f) show the C 1s and O 1s XPS spectra measured in ultrahigh vacuum after evacuating CO2 gas. After gas evacuation, neither adsorbed CO2 nor gas-phase CO2 were observed. Therefore, CO2 molecules were only present on graphene under near-ambient pressure gas at 175 K. When CO2 molecules were adsorbed on the graphene surface, additional XPS features were observed at 532.0 eV in O 1s and 286.7 eV in C 1s XPS spectra (Figs. 1(c, d)). These features were assigned to epoxy (C-O-C) group on graphene. The photo-induced dissociation of CO2 molecules (CO2→CO + O) at high X-ray photon flux causes the formation of epoxy groups on graphene. The oxygen-functionalized graphene surface binds CO2 molecules more strongly than the pristine graphene surface.
The increase in the adsorption energy of CO2 on the oxygen-functionalized graphene surface was further investigated by first-principles calculations with the van der Waals density functional (vdW-DF) method. The adsorption energy of CO2 was increased by ~5 kJ/mol from 20.2 kJ/mol on the pristine graphene surface [2] to 25.7 kJ/mol on the oxygen-functionalized graphene surface. This was in good agreement with the experimental value (≥ 5 kJ/mol) derived from an adsorption and desorption equilibrium relationship. In addition, first-principles calculations revealed that the most stable adsorption site of CO2 on the oxygen-functionalized graphene surface was not on top of the epoxy group, but on the C-C bond of graphene adjacent to the epoxy group (Fig. 2). The adsorption of CO2 on the oxygen-functionalized graphene surface was stabilized by both the electrostatic interactions between the CO2 and epoxy group and the vdW interactions between the CO2 and graphene. The detailed understanding of the interaction between CO2 and the oxygen-functionalized graphene surface obtained in the present study may assist in developing guidelines for designing novel graphene-based catalysts.
References
- [1] S. Yamamoto, K. Takeuchi, Y. Hamamoto, R.-Y. Liu, Y. Shiozawa, T. Koitaya, T. Someya, K. Tashima, H. Fukidome, K. Mukai, S. Yoshimoto, M. Suemitsu, Y. Morikawa, J. Yoshinobu, and I. Matsuda, Phys. Chem. Chem. Phys. 20, 19532 (2018).
- [2] K. Takeuchi, S. Yamamoto, Y. Hamamoto, Y. Shiozawa, K. Tashima, H. Fukidome, T. Koitaya, K. Mukai, S. Yoshimoto, M. Suemitsu, Y. Morikawa, J. Yoshinobu, and I. Matsuda, J. Phys. Chem. C 121, 2807 (2017).
