Construction of Three-Dimensional Anionic Molecular Frameworks Based on Hydrogen-Bonded Metal Dithiolene Complexes and the Crystal Solvent Effect
Mori Group
Metal organic frameworks (MOFs) and covalent organic frameworks (COFs) are a class of crystalline materials with highly periodic structures formed through coordination or covalent bonds. Extensive synthetic studies of these materials have been carried out, because of the uniqueness of their structures and the resulting functionalities, such as gas adsorption/separation, catalytic, and sensing abilities and fuel cell properties. In addition, hydrogen-bonded (H-bonded) organic frameworks (HOFs) have recently emerged as a new class of such crystalline materials. By utilizing H-bonds instead of coordination or covalent bonds, HOFs have generally better crystallinity, solution processability, and flexibility than MOFs and COFs. Such ionic frameworks are expected to show unique features different from conventional neutral ones, due to their intrinsic ionic nature and the accompanying cation–anion interactions. However, the number of ionic frameworks reported so far is limited.
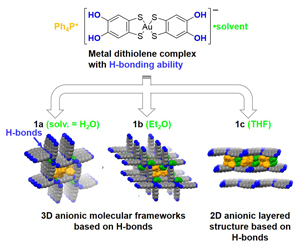
Fig. 1. On the basis of the design and synthesis of an anionic gold dithiolene complex [Au(catdt)2]− with four hydroxyl groups, we have successfully constructed the first 3D molecular frameworks based on H-bonded metal dithiolene complexes, (Ph4P)[Au(catdt)2]·0.5H2O (1a) and (Ph4P)[Au(catdt)2]·Et2O·n(solv) (1b; solv = Et2O and/or acetone) as well as 2D layered structure of (Ph4P)[Au(catdt)2]·2THF (1c). The key for constructing the 3D frameworks are (i) connecting the planar [Au(catdt)2]− molecules with 3D intermolecular H-bonds and (ii) introducing large Ph4P+ cations into the channels. Interestingly, metal complexes are highly influenced by the solvent molecules included in the crystals (especially, the molecular size and shape and H-bonding abilities), which leads to variations in the assembled structure of the [Au(catdt)2]− molecules; namely, a 3D framework with densely packed walls in water containing 1a, a 3D framework with loosely packed walls in ether-containing 1b, and a 2D layered structure in THF containing 1c.
Recently, we have designed and synthesized a novel metal dithiolene complex with hydrogen-bonding (H-bonding) ability, [Au(catdt)2]− (catdt: catechol-4,5-dithiolate) and successfully constructed [Au(catdt)2]−-based three dimensional (3D) anionic molecular frameworks in two kinds of salts, (Ph4P)[Au(catdt)2]·0.5H2O (1a) and (Ph4P)[Au(catdt)2]·Et2O·n(solv) (1b; solv = Et2O and/or acetone). To our knowledge, these are the first examples of the 3D molecular frameworks based on H-bonded metal dithiolene complexes. Importantly, these frameworks are constructed through 3D intermolecular H-bonds between [Au(catdt)2]− molecules, and have tubular channels occupied by large Ph4P+ cations, where multiple cation–anion short contacts are formed to stabilize the 3D framework structures. Furthermore, in addition to 1a and 1b with 3D frameworks, we have obtained (Ph4P)[Au(catdt)2]·2THF (1c) having a 2D layered structure. A detailed comparison of the structures of these compounds reveals that the included solvent molecules play an important role in regulating the intermolecular interactions and assembled structures. Interestingly, we have found that, due to the differences in the H-bonding ability and molecular size of H2O and Et2O, the wall structures of the 3D frameworks in 1a without voids and 1b with voids are qualitatively different. [1]
Three kinds of 1:1 salts of Ph4P+ and [Au(catdt)2]−, that is, (Ph4P)[Au(catdt)2]·0.5H2O (1a), (Ph4P)[Au(catdt)2]·Et2O·n(solv) (1b; solv = Et2O and/or acetone), and (Ph4P)[Au(catdt)2]·2THF (1c), were synthesized, and their structures and chemical compositions were determined by single crystal X-ray analysis. Recrystallization of the obtained powder from acetone/hexane, acetone/Et2O or THF/hexane provided the salt 1a (containing H2O) as yellowish brown needle-like crystals, 1b (containing Et2O and/or acetone) as yellow plate-like crystals or 1c (containing THF) as green plate-like crystals, respectively.
We first compare the structures of three kinds of novel [Au(catdt)2]−-based salts, (Ph4P)[Au(catdt)2]·0.5H2O 1a, (Ph4P)[Au(catdt)2]·Et2O·n(solv) (1b; solv = Et2O and/or acetone), and (Ph4P)[Au(catdt)2]·2THF (1c). All of them are 1:1 salts of Ph4P+ and [Au(catdt)2]−; however, their crystal structures are qualitatively different from each other, depending on the solvent molecules included in the crystal. The most prominent difference is the dimensionality of the assembled structures of [Au(catdt)2]−; namely, in the water and ether-containing salts 1a and 1b, this planar anionic molecule forms a 3D assembled structure (i.e., 3D framework, Fig. 1), whereas in the THF-containing salt 1c, it forms not a 3D but a 2D structure (i.e., 2D layers). Furthermore, although both the 3D framework structures in 1a and 1b have tubular channels with a similar size, the structures of their walls are significantly different from each other; namely, the walls in 1a are densely packed with [Au(catdt)2]−, whereas those in 1b are loosely packed to form voids, into which the cation and Et2O molecules partially penetrate (Fig. 1). These differences should be caused by changing the intermolecular interactions between the component molecules upon the change of the crystal solvent.
We finally discuss how the crystal solvent makes differences in the intermolecular interactions and assembled structures. Here, we focus on the H-bonding ability, size, and shape of the crystal solvents. Et2O, included in the 3D system 1b, and THF, included in the 2D system 1c, have proton- accepting ability due to the ether oxygen atom, which actually allows them to form the H-bonds with the catechol O–H protons of [Au(catdt)2]− molecules. On the other hand, the overall shapes of these solvent molecules are greatly different from each other [rod-shaped (Et2O) vs. disk-shaped (THF)], which should contribute to the great structural difference in 1b and 1c (i.e., 3D vs. 2D). Namely, due to the disk shape, the THF molecules are positionally (orientationally) disordered, which leads to the formation of the THF–THF H-bonds and consequently the 2D layered structure. In contrast, the rod-shaped Et2O molecules have no such structural disorder and additional intermolecular interactions; thus they seem to simply occupy the space between the large Ph4P+ and [Au(catdt)2]− molecules without interrupting the intermolecular interactions between the Ph4P+ and [Au(catdt)2]− molecules for constructing the 3D framework. Meanwhile, due to the steric bulkiness of the ethyl groups in Et2O, the walls of the 3D framework have the voids. In contrast, the water containing 3D system 1a has no such voids and thus the wall is densely packed with the lateral S⋯S interactions between [Au(catdt)2]− molecules. This is probably because the solvent water molecules are involved in the wall structure, which should eliminate the above mentioned steric hindrance effect from the solvent molecules. Here, the water molecule has both proton-donating and -accepting abilities, in contrast to Et2O and THF having only proton-accepting ability. In addition, the size of water molecule is much smaller than that of the others. These features should allow the water molecules to exist between the catechol moieties of [Au(catdt)2]− molecules and participate in the densely packed wall structure of the 3D framework. Therefore, we have successfully illustrated the role and effect of the crystal solvent molecules on the construction of these new 3D anionic molecular frameworks based on [Au(catdt)2]− in terms of the molecular size and shape and H-bonding abilities. As for the next step, by taking advantage of this new type of metal dithiolene complex with H-bonding abilities, we are currently investigating cooperative properties and functionalities of H-bond dynamics (e.g. dielectric properties and proton conductivity) and π−/d-electrons (e.g. electronic conductivity and magnetic properties).
References
- [1] S. Yokomori, A. Ueda, T. Higashino, R. Kumai, Y. Murakami, and H. Mori, CrystEngCommun, 21, 2940 (2019).
