Thermodynamic and Structural Studies on Super High Entropy Liquids, Alkylated Tetra-phenyl Porphyrins
Yamamuro Group
The fusion (melting) temperature Tfus of molecules usually depend on molecular mass M; the larger M is, the higher Tfus becomes. For example, Tfus of benzene (C6H6, M = 78) is 279 K while that of biphenyl (C6H5-C6H5, M = 154) is 342 K. This is because the intermolecular van der Waals interaction is larger in the crystalline phase with better molecular packing than that in the liquid phase with worse packing.
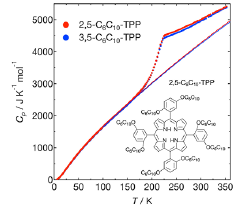
Fig. 1. Heat capacities of 2,5-C6C10-TPP and 3,5-C6C10-TPP. The inset shows the molecular structure of 2,5-C6C10-TPP.
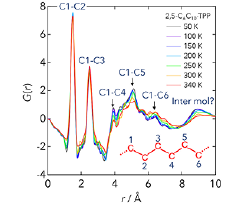
Fig. 2. Temperature dependence of the reduced pair distribution functions of 2,5-C6C10-TPP calculated from the X-ray diffraction data. The distances corresponding to the intra and intermolecular correlations are given in the figure.
Recently, Nakanishi group in NIMS found a series of large molecules which exist in liquid states at room temperature. These molecules have π-conjugated cores such as pyrene, naphthalene, anthracene, fullerene, phthalocyanine and long alkylchains bonded to the cores [1]. We consider that these alkylated molecules are stabilized by the large entropy effect which is caused by the conformational disorder of alkylchains. This situation is similar to that of ionic liquids which are in liquid states in spite of their strong inter-ionic interactions. We collectively call this type of liquids “super-high entropy liquids (SHEL)”. The physico-chemical properties of SHEL have not been studied well. As the first target we have taken 2,5-C6C10-tetraphenylporphyrin (2,5-C6C10-TPP) and 3,5-C6C10-tetraphenylporphyrin (3,5-C6C10-TPP). The molecular structure of 2,5-C6C10-TPP is shown by the inset of Fig. 1; two -O-C6C10 groups are bonded symmetrically to a benzene ring in 3,5-C6C10-TPP. It is quite interesting that Tfus of the alkylated molecules (2,5-C6C10-TPP, M = 2538) is lower than Tfus of non-alkylated molecules (TPP, M = 615, Tfus = 723 K).
We have measured the heat capacities of 2,5-C6C10-TPP and 3,5-C6C10-TPP by use of an adiabatic calorimeter in our lab. The Cp plot shown in Fig. 1 revealed that both molecules have a broad glass transition at around Tg = 210 K and the Cp of 2,5-C6C10-TPP is 2-3 % larger than that of 3,5-C6C10-TPP at above Tg. The configurational entropies calculated from the Cp data are more than 1000 JK-1mol-1 at high temperature limit, which is more than 10 times larger than those of usual molecular liquids. We have also measured the X-ray diffraction of 2,5-C6C10-TPP and 3,5-C6C10-TPP using a diffractometer at BL04B2, SPring-8. Figure 2 shows the reduced pair-correlation function G(r) calculated from the diffraction data; a similar result is obtained in 3,5-C6C10-TPP. Most of the G(r) peaks are attributed to the C-C correlations in alkylchains. The broad peak at 9 Å, maybe also at 4.5 Å, is considered to be the correlation between porphyrin rings. The present data suggest that the configurations of alkylchains and porphyrin rings of 2,5-C6C10-TPP and 3,5-C6C10-TPP are highly disordered at higher temperatures and becomes ordered at lower temperatures. Now we are measuring the quasielastic neutron scattering of both samples to investigate the dynamics of both alkylchains and porphyrin cores.
References
- [1] A. Ghosh and T. Nakanishi, Chem. Commun. 53, 10344 (2017).
- [2] A. Ghosh, M. Yoshida, K. Suemori, H. Isago, N. Kobayashi, Y. Mizutani, Y. Kurashige, I. Kawamura, M. Nirei, O. Yamamuro, T. Takaya, K. Iwata, A. Saeki, K. Nagura, S. Ishihara, and T. Nakanishi, Nature Chem., submitted.
