Biophysical Study on the Function and Molecular Mechanism of Rhodopsins
Inoue Group
Rhodopsin is photo-receptive heptahelical transmembrane proteins in which retinal chromophore is covalently bound to a conserved lysine residue in the seventh helix, and animal and microbial rhodopsin families are known so far. Animal rhodopsins present in animal retina transfer visual signal to brain and are also related to non-visual light sensing. Microbial rhodopsins show diverse functions: light-driven ion pump, light-gated ion channels, light-regulated enzyme and so on. Both types of rhodopsins are being widely used in optogenetics to control various cellular events, such as neural activity, gene expression and so on, by light. Recently, we reported third class of rhodopsin, heliorhodopsin (HeR), which is evolutionally isolated from both of microbial and animal rhodopsins [1]. The biochemical study revealed its inverted structure compared with other class of rhodopsins, and N- and C-termini of HeR faces extracellular and cytoplasmic milieus, respectively (Fig. 1). HeRs are diversely distributed from bacteria, archaea, eukaryotic algae and giant viruses. Although the function of HeR has not been clarified yet, we observed a long photo-reaction cycle up to seconds to minutes by laser flash photolysis suggesting HeR works as a signaling protein which transfers light signal to unidentified intracellular proteins. The retinal chromophore in HeR isomerizes from all-trans to 13-cis form upon light illumination, and we dominantly observed K, M and O-intermediates in the photocycle. While the K and O intermediates show red-shifted absorption compared with that of the dark state, the absorption of M-intermediate is highly blue-shifted to 400 nm and a proton is released from retinylidene Schiff-base. A mutational study suggested that the proton is not transferred to a specific residue and it is trapped in a hydrogen bonding network, called proton accepting group (PAG), constituted from several amino-acid residues including conserved histidines and internal water molecules on the extracellular side of the protein.
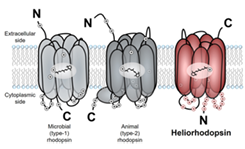
Fig. 1. Molecular structures of microbial rhodopsin, animal rhodopsin and heliorhodopsin.
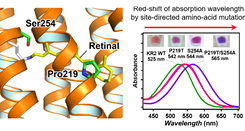
Fig. 2. Construction of light-driven sodium pump rhodopsin absorbing longer-wavelength light by site-directed amino-acid mutagenesis
Rhodopsins show wide range of absorption wavelength and the color regulation of retinal chromophore in rhodopsins to achieve longer-wavelength absorption is one of critical elemental technique to develop new types of optogenetics tools which can avoid strong light-scattering by biological tissues and phototoxicity. We reported that the mutation of the conserved proline and serine residues in sodium pump rhodopsin (KR2) induces 40-nm red-shift of the absorption without impairing sodium-transport activity [2]. Also, FTIR spectroscopy and quantum mechanical calculation revealed that the change in dipole moments of these residues lowered energy gap of π-electron of retinal chromophore by electrostatic interaction. Furthermore, we identified a new type of sodium pump rhodopsin from α-proteobacterium, Jannaschia seosinensis, in which the proline residue was naturally altered to glycine and it shows 20-nm longer absorption than that of KR2. Since J. seosinensis is derived from solar saltern, the natural mutation of the proline occurred to use longer wavelength light in turbid environment. Since sodium pump rhodopsin can suppress the neural spiking without alterations of intracellular pH and Cl- concentration, this red-shifted KR2 would be expected to be applied as a new type of optogenetic tool.
References
- [1] A. Pushkarev, K. Inoue et al., Nature 558, 595 (2018).
- [2] K. Inoue, M. d. C. Marín et al., Nat. Commun. 10, 1993 (2019).
