CO2 Activation and Reaction on Zn-Deposited Cu Surfaces Studied by Ambient-Pressure X-Ray Photoelectron Spectroscopy
Yoshinobu and I. Matsuda Groups
It is important to understand the mechanisms of activation and hydrogenation of carbon dioxide (CO2) in order to utilize the abundant CO2 effectively as a chemical feedstock. One way of utilizing CO2 is methanol synthesis on Cu-based catalysts. Metallic copper in the catalysts is considered to be an active site for methanol synthesis. So far, the adsorption and reaction of CO2 on Cu surfaces have been widely investigated under ultrahigh vacuum (UHV) and under ambient pressure conditions.
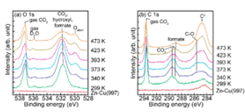
Fig. 1. A series of XPS spectra on Zn(0.25 ML)/Cu(997) surface: (a) O 1s and (b) C 1s. The spectra of bare Zn-Cu(997) surface were measured under UHV. AP-XPS measurements were first performed at 299 K in the presence of 0.8 mbar CO2, 0.4 mbar H2, and 0.05 mbar D2O, then the sample was heated up to 473 K under this ambient-pressure condition.
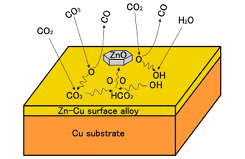
Fig. 2. Schematic diagram of proposed reactions on the Zn-deposited Cu surface in the presence of CO2, H2 and H2O.
In this study [1], the reaction of CO2 on Zn-Cu(111) and Zn-Cu(997) was systematically studied using ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) at SPring-8 BL07LSU (2015B7496 and 2016A7401). AP-XPS allows adsorbate and substrate electronic states to be elucidated under reaction conditions. The aim of this study is to reveal the roles of Zn and water in the CO2 reaction, and elementary steps of methanol synthesis by a series of systematic experiments performed under well-defined conditions at temperature between 299-473 K. We used Zn-deposited Cu(111) and Cu(997) single crystals as model catalytic systems. Stepped Cu surfaces are more reactive for dissociation of CO2 compared to flat Cu surfaces. Recently, we have investigated adsorption states of CO2 on Cu(997) surfaces under UHV, and near-ambient condition. In this study, we have focused on hydrogenation of CO2 under near-ambient pressure conditions to clarify the effect of the step sites. The oxidation state of Zn-deposited Cu surfaces and the stability of reaction products depend strongly on the gas composition and sample temperature. The effect of water on the CO2 activation was also examined by a set of control experiments.
In the presence of 0.8 mbar CO2 and 0.4 mbar H2 gases, hydrogenation products are not observed; only carbonate is formed on Zn-deposited Cu(111) and Cu(997) surfaces (not shown here). We found that the formation rate of carbonate at 299 K is significantly faster on Zn/Cu(997) than that on Zn/Cu(111), indicating that step sites are more reactive for CO2 activation than terrace sites. On the other hand, the addition of water (D2O) in the feed gas leads to hydrogenation of CO2 to formate at sample temperatures around 400 K (Fig. 1). This suggests that hydroxyl produced from dissociative adsorption of water is a reactant in the CO2 hydrogenation observed under the present reaction conditions. Figure 2 schematically shows the proposed reactions on the Zn-deposited Cu surface in the presence of CO2, H2 and H2O. We also found that the reaction products such as carbonate and formate on the Zn/Cu(997) surface are more stable than those on the bare Cu(997) surface. In particular, formate remains on Zn-deposited Cu surfaces up to 473 K, which is close to the operation temperature of industrial Cu-ZnO catalysts. We conclude that an important role of Zn on Cu surfaces is the stabilization of reaction intermediates originated from CO2.
References
- [1] T. Koitaya, S. Yamamoto, Y. Shiozawa, Y. Yoshikura, M. Hasegawa, J. Tang, K. Takeuchi, K. Mukai, S. Yoshimoto, I. Matsuda, and J. Yoshinobu, ACS Catal. 9, 4539 (2019).
