Noble Metal Segregation in SrTiO3
M. Lee and M. Lippmaa
Photoelectrochemical water splitting is a process where photoexcited electrons and holes are used to drive a chemical hydrogen or oxygen evolution reaction at the surface of a semiconductor photoelectrode. The technique can be used to harvest solar energy and store the energy in the form of hydrogen fuel. In the semiconductor materials studied so far, the solar-to-hydrogen conversion process efficiency is still too low for practical applications. Oxide semiconductors such as titanates are often used as the electrode materials due to the chemical stability in water, but the energy conversion efficiency is limited by the loss of photocarriers to trapping and recombination. A general solution to this problem is to construct a nanostructured photoelectrode where light can be efficiently absorbed but a short escape path exists for the photocarriers to reach the surface before recombination can occur. For example, an array of semiconductor nanowires can be used, which would increase the effective surface area, guarantees a short carrier escape path in the radial direction of a wire, while still efficiently absorbing incident sunlight. A drawback of this strategy is the complicated synthesis process of nanowires and limited long-term mechanical stability. We have therefore studied the possibility of using spontaneous noble metal segregation in a perovskite lattice to embed self-organized nanoscale metal electrodes in the bulk of an oxide semiconductor. We have demonstrated the formation of Ir nanopillars in SrTiO3 thin films and photoelectrochemical measurements have shown that embedding metal electrodes in a semiconductor does indeed improve the energy conversion efficiency [1]. The main benefit of this structure is the simple one-step synthesis process and mechanical robustness.
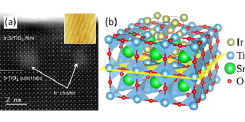
Fig. 1. (a) Cross-sectional HAADF-STEM image of a Ir:SrTiO3 film on a SrTiO3 substrate showing the formation of bright Ir clusters at the film interface. (inset) AFM image of the film surface, showing a smooth step-and-terrace morphology. (b) Structural model of a lattice-matched fcc Ir metal inclusion in the SrTiO3 lattice.
The purpose of this joint-use project was to study the initial stage of Ir metal segregation in SrTiO3 thin films [2]. As shown in the inset of Fig. 1a, the surface morphology of thin Ir:SrTiO3 (~5 unit cell) films imaged by atomic force microscopy (AFM) shows no obvious metal segregation effects. However, bright clusters are observed in high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images. An in-situ energy-dispersive x-ray spectroscopic analysis showed that the bright clusters in the film are strongly enriched in Ir and consistent with a face-centered cubic (fcc) metal cluster embedded in the perovskite lattice, as illustrated in Fig. 1b. It is clear from the STEM analysis that the clusters have uniform lateral size of about 2 × 2 perovskite unit cells. The thin films were grown at strongly reducing conditions, which means that the perovskite lattice contains oxygen vacancies, promoting the substitution of Ir atoms at the anion site. The anion substitution allows the fcc metal to form a lattice-matched metal inclusion in the SrTiO3 lattice. When similar films are grown at slightly more oxidizing conditions, extended Ir metal nanopillars form instead of the isolated metal clusters. This work helps to design more elaborate oxide metal nanocomposites for energy harvesting applications.
References
- [1] S. Kawasaki, R. Takahashi, T. Yamamoto, M. Kobayashi, H. Kumigashira, J. Yoshinobu, F. Komori, A. Kudo, and M. Lippmaa, Nat. Commun. 7, 11818 (2016).
- [2] M. Lee, R. Arras, R. Takahashi, B. Warot-Fonrose, H. Daimon, M.-J. Casanove, and M. Lippmaa, ACS Omega 3, 2169 (2018).
