Enhancement of the Hydrogen-Bonding Network of Water Confined in a Polyelectrolyte Brush
Harada Group
In the crowded environment of a living cell, several biomolecular polyelectrolytes−especially proteins, nucleic acids, and complex sugars−are compressed together. Water encapsulated between the aforementioned entities is no longer a simple space-filling medium but is believed to exhibit intriguing hydrogen-bonding networks depending on its molecular dimensions and interfacial properties. This unique hydrogen-bonding structure of water in the vicinity of polyelectrolytes should significantly affect the specific structure and functions of biomolecules and their assemblies. Hence, it is imperative to devise a novel method for investigating the local hydrogen-bonding structure of water near polyelectrolytes, which can aid in the understanding of the functions of biological systems. A polyelectrolyte brush, a surface-tethered polymer layer is a proper model interface of polymeric soft materials, such as proteins. A recent study using microscopic infrared (IR) spectroscopy indicated that water molecules are confined in a polyelectrolyte brush with a highly ordered hydrogen-bonding network. The formation of a hydration layer of polyelectrolytes and the resulting hydrogen-bonding network is expected to be crucial to controlling the properties of biomolecules, such as lubrication and antifouling. This study sheds light on the local hydrogen-bonding structure of water confined in a charged polyelectrolyte brush and reveals that the hydrogen-bonding structure of water confined in the polyelectrolyte brush is very similar to ice Ih, even at room temperature [1].
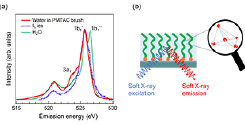
Fig. 1. (a) O 1s soft X-ray emission spectra of water confined in the polyelectrolyte brush, the dry brush measured in vacuum, liquid H2O, and ice Ih. The excitation energy is 550.3 eV, which is well above the ionization threshold. (b) Schematic of the hydrogen-bonding structure of water confined in the PMTAC brush.
O 1s X-ray emission spectroscopy (XES) experiments of water confined in the polyelectrolyte brush were performed at the SPring-8 synchrotron radiation facility using the BL07LSU HORNET station. Figure 1(a) compares the O 1s XES spectra of the water confined in the poly[2-(methacryloyloxy)- ethyltrimethylammonium chloride] (PMTAC) brush, bulk liquid water, and ice Ih. The XES spectra of the bulk liquid water and ice Ih are shown with the same 1b1 peak intensity as that of confined water to compare the spectral shape. The XES spectra of the confined water and ice Ih are very similar; the 1b1" peak, which is observed in the spectrum of bulk liquid water, is negligible. The similarity between the two XES spectra is quite surprising as a significant enhancement of hydrogen-bonding is observed even at room temperature in the water confined in the PMTAC brush. Moreover, enhanced spectral intensity is observed around the 3a1 region for the confined water as compared to that in ice Ih and bulk liquid water. The 3a1 peak, which is attributed to the dipole-forbidden transition (3a1 → 1a1) in tetrahedral symmetry, is not observed for ice Ih and is smeared out in the case of bulk liquid water because of the high sensitivity of the 3a1 orbital to the distribution of the hydrogen-bond strength. Therefore, the enhancement of the 3a1 peak observed for the confined water can be explained by the fact that the hydrogen bonds are distorted (not straight O−H···O bonds), albeit somewhat ordered, in the PMTAC brush.
Our XES results confirm that these techniques are extremely sensitive to the presence/absence and distortion of hydrogen-bonding. A majority of water molecules in the polyelectrolyte brush are held together by one type of hydrogen-bonding configuration: possibly a slightly distorted but ordered hydrogen-bonding configuration even at room temperature. The distorted picture is only a speculation from the emergence of the 3a1 state and the consideration of the possible hydrogen-bond structure in the vicinity of the charged site, which needs further study from both experimental and theoretical aspects. We believe that XES will pave the way for novel, important research fields in the near future.
References
- [1] K. Yamazoe, Y. Higaki, Y. Inutsuka, J. Miyawaki, Y. –T. Cui, A. Takahara, and Y. Harada, Langmuir 33, 3954 (2017).
