Evidence of Charge Transfer and Orbital Magnetic Moment in the Multiferroic CuFeO2
Y. Narumi, T. Nakamura, and H. Ikeno
Original meaning of “multiferroics” is coexistence of “multiple” and “ferroic” ordered states. However, nowadays, multiferoics is commonly recognized as phenomena involving antiferroic properties such as antiferromagnetic. As a result, multiferroics comes to have diversity in functionality, such as magnetic-field-control of ferroelectricity. The triangular lattice antiferromagnet CuFeO2 (abbreviated to CFO) shows ferroelectricity in a finite magnetic field [1]. Nominal oxidation state of Fe is Fe3+ (3d5) with spin S = 5/2 and with orbital singlet L = 0. Therefore a classical ordered state is expected to be a noncollinear 120 degrees spin arrangement. However, in reality, CFO shows a 4-sublattice collinear magnetic ordering below 11 K by forming a scalene triangle, because of the geometrical frustration and a spin-lattice coupling. By an application of magnetic field, CFO shows successive magnetization jumps, although no magnetic anisotropy is expected. The ferroelectricity can be explained by a recently proposed mechanism, d-p hybridization, in which a spin-dependent charge transfer (CT) between the transition metal and the ligand is essential [2].
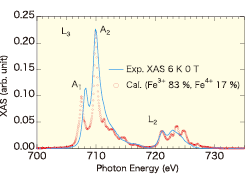
Fig. 1. Experimental (blue solid line) and theoretical (red open circle) XAS's of Fe L2,3-edge of CFO at 6 K without magnetic field. The data are offset and amplified by a numerical factor so as to scale them at the pre-edge and the maximum of L3-edge.
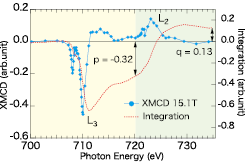
Fig. 2. Experimental XMCD of Fe L2,3-edges at 15.1 T (blue solid line with solid circle) and integration of it (red dashed line). According to Sum rule, the ratio of spin to orbital magnetic moments is given by ms/mo = 2q/(9q - 6q). The numerical values of p and q indicated by arrows are the integrals of the XMCD over the L3 and the whole L2,3 absorption regions, respectively.
The aim of the present study is to clarify the local electronic state of CFO, which should play an important role in the ferroelectricity and the magnetic anisotropy. For that reason, we performed x-ray magnetic circular dichroism (XMCD) measurements in high magnetic fields at SPring-8/BL25SU [3]. By tuning an incident energy to the absorption edge of a specific magnetic ion, XMCD gives us the spin and orbital magnetic moments of the ions independently. Moreover the x-ray absorption spectrum (XAS) is sensitive to the valence state of the ion. Therefore we are able to investigate both magnetic and electronic properties from the microscopic point of view. The ultra-high vacuum chamber equipped with a pulse magnet was developed in collaboration with Tohoku University, SPring-8, and University of Tokyo. This is the unique soft x-ray spectrometer reaching 40 T in the world.
Figure 1 shows the comparison between the experimental XAS of Fe L2,3-edge at 6 K and the ab-initio multiplet calculation at 0 T. The broadness of the experimental XAS is not due to resolution. It is because that there is a restriction of the number of the electronic configurations in the calculations. There is unignorable discrepancy with respect to the energy split between main A2 and satellite A1 peaks. This is also due to the same reason as the fine structures in the theoretical calculation. Although nominal valence of Fe is Fe3+, an addition of a small amount of Fe4+ component leads to a better agreement than the theoretical XAS of only Fe3+. Figure 2 represents experimental results of Fe L2,3-edge XMCD at 15.1 T and corresponding energy-integral. By analysis based on sum rule, the ratio of spin (ms) to orbital (mo) magnetic moments was determined to be ms/mo = -0.071. That is, the ms and mo point in the opposite direction to each other.
According to Hund's rules, the antiparallel configuration of the ms and mo leads to the fact that the 3d electronic configuration of Fe should be less than half-filled. In other words, we can say that Fe4+ (3d4) state exists. From the viewpoint of charge compensation among ions, presence of Fe4+ ion needs a CT from somewhere. Accordingly, we measured XAS of Cu L2,3-edges and related compounds for comparison. Surprisingly, it turned out that the valence state of Cu is similar to neither that of Cu+ nor that of Cu2+, but that of Cu metal. This gives a new insight that it is important to take into consideration for correlation between Fe and Cu as well as oxygen in order to fully understand ferroelectric and magnetic properties of CFO.
References
- [1] T. Kimura, et al., Phys. Rev. B 73, 220401(R) (2006).
- [2] T. Arima, J. Phys. Soc. Jpn. 76, 073702 (2007).
- [3] Y. Narumi, et al., J. Phys. Soc. Jpn. 85, 114705 (2016).
