Improved Stability of a Metallic State in Benzothienobenzothiophene (BTBT)-based Molecular Conductors: an Effective Increase of Dimensionality with Hydrogen Bonds
Mori Group
One of the merits in organic conductors based upon molecules is the designability and variety of molecular components [1], which lead a wide range of electronic states such as exotic metallic [2], superconducting [3], Dirac Fermion, quantum spin liquid [4], and electron glass states. These curious solid states [2-6] have been given a birth based upon newly designed and synthesized molecules, usually tetrathiafulvalene (TTF) and its derivatives with 7 π system. Recently, another donor molecule benzothienobenzothiophene (BTBT) with 6 π system has been synthesized to be a superior semiconductor for field-effect-transistor. Although this BTBT is poor electron donor, it is realized to afford one-dimensional molecular conductor, (BTBT)2PF6. In this article, the successfully improved stability of a metallic state in newly synthesized BTBT derivative-based molecular conductor, β-[BTBT(OH)2]2ClO4, by the increase of dimensionality with hydrogen (H)-bonds is reported [6].
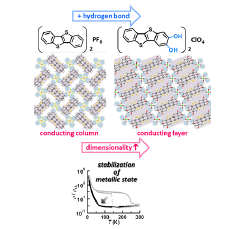
Fig. 1. We have proved that hydrogen-bonding interaction can increase the dimensionality and stabilize a metallic state for the newly synthesized benzothienobenzothiophene (BTBT)-based molecular conductor, β-[BTBT(OH)2]2ClO4. This charge-transfer complex offers a new promising strategy for designing and developing next generation organic electronic materials/devices.
The novel donor molecule BTBT(OH)2 was synthesized by 7 steps with utilizing Sonogashira coupling method. Surprisingly, our newly synthesized BTBT(OH)2 functionalized at the 2,3-positions has been unknown, although a lot of BTBT derivatives have been designed and synthesized so far. Therefore, our present synthetic strategy will be effective in exploring a new class of functionalized BTBT derivatives. The electrocrystallization of BTBT(OH)2 in the presence of tetra-n-butylammonium perchlorate gave the molecular conductor, needle-like black crystals of the ClO4 salt, namely β-[BTBT(OH)2]2ClO4. The BTBT(OH)2 is partially oxidized state with a +0.5e charge and expected to form a 3/4-filled band structure. The BTBT(OH)2 molecule is almost planar and forms a head-to-tail-type uniform stack along the b-axis with an inter-planar spacing of 3.326 Å. It is noteworthy that two kinds of [O–H..O]-type H-bonding interactions were observed between the hydroxy groups of the donor and the ClO4 anion. Consequently, an infinite 1D H-bond-chain structure is formed along the c-axis. In this arrangement, very weak C–H..S interactions are also found in the side-by-side direction of the donor molecule. As a result of these intermolecular interactions, BTBT(OH)2 produces a sheet type molecular arrangement (Fig. 1). The transfer integrals, which correspond to inter-molecular interactions between the neighbouring molecules, are largest in the stacking direction b (91.0 meV) and relatively smaller in the diagonal directions p (2.77 meV), q (13.6 meV), and r (13.1 meV). The effect of the H-bond interactions in the crystal of β-[BTBT(OH)2]2ClO4 is further disclosed by comparing the crystal structures of β-[BTBT(OH)2]2ClO4 and the parent salt (BTBT)2PF6. As shown in Fig. 1, the BTBT molecules in (BTBT)2PF6 form windmill-type columnar structures with effective π–π interactions. There are, however, no effective interactions between the columns, due to the existence of C–H..F contacts between the donor molecule and the PF6 anion. As a result, the columnar arrangement produces a typical 1D electronic structure with a flat Fermi surface. On the other hand, the π-stacking columns in the present salt β-[BTBT(OH)2]2ClO4 (Fig. 1) are connected with the O–H..O H-bonding interactions through the ClO4 anions. The resultant Fermi surface is warped in the 3/4-filled band structure, which means the formation of a quasi-one-dimensional (Q1D) electronic structure in β-[BTBT(OH)2]2ClO4. This enhancement of the electronic structure from 1D to Q1D is also evidenced by comparing the anisotropy of the transfer integrals. (BTBT)2PF6 has a strong interaction within the π-stacking column (87 meV); however, the interstack interaction is negligibly small (1.4 meV). On the other hand, the present β-[BTBT(OH)2]2ClO4 has the substantial interstack interactions (q, r ~ 13 meV), in addition to the strong intrastack one (b = 91 meV), as described before. Thus, the intrastack/interstack anisotropy is significantly decreased from 60 (= 87/1.4) in (BTBT)2PF6 to 7 (= 91/13) in β-[BTBT(OH)2]2ClO4.
The increase of the dimensionality of the electronic structure significantly influenced the electrical conducting properties. The crystal of β-[BTBT(OH)2]2ClO4 shows a relatively low room temperature electrical resistivity (ρ300K = 5.5 × 10-3 ohm cm), which decreases with decreasing temperature down to 135 K. This temperature dependence indicates that this salt is metallic above 135 K, and more importantly, this metallic state is more stable than that of (BTBT)2PF6. This is because this salt does not show an abrupt resistivity jump, as seen in (BTBT)2PF6 at 150 K. Therefore, we have proved that the increase of the dimensionality caused by the H-bond interactions brings about the stabilization of the metallic state in BTBT-based conductors. On further cooling, this salt finally undergoes a metal–insulator-like transition around 60 K, after entering the semiconducting state at 135 K. A similar transition without hysteresis has also been observed in (BTBT)2PF6 at around 50 K.
In conclusion, we have successfully synthesized novel organic donor with 6 π system and the introduction of hydrogen bond, benzothienobenzothiophene (BTBT) derivative, BTBT(OH)2, and realized a stable metallic state in a quasi-one dimensional charge-transfer salt, β-[BTBT(OH)2]2ClO4. The strong H-bonding ability of the catechol-type hydroxyl groups has played a crucial role in the formation of an infinite one-dimensional H-bonded chain structure, which leads to the increase of the dimensionality of the electronic structure and the stable metallic state. These results demonstrate that functionalized BTBT derivatives are promising electron donors in molecular conductors. We believe that this study will pave a new way for designing and developing high-dimensional BTBT-based materials/devices with interesting conducting properties (e.g. superconductivity and high carrier mobility).
References
- [1] H. Kamo, A. Ueda, T. Isono, K. Takahashi, and H. Mori, Tetrahedron Lett. 53, 4385 (2012).
- [2] T. Isono, H. Kamo, A. Ueda, K. Takahashi, A. Nakao, R. Kumai, H. Nakao, K. Kobayashi, Y. Murakami, and H. Mori, Nature Commun. 4, 1344 (2013).
- [3] S. Kimura, R. Chiba, T. Mori, T. Kawamoto, H. Mori, H. Moriyama, Y. Nishio, and K. Kajita, Chem. Commun. 2004, 2454.
- [4] T. Isono, H. Kamo, A. Ueda, K. Takahashi, M. Kimata, H. Tajima, S. Tsuchiya, T. Terashima, S. Uji, and H. Mori, Phys. Rev. Lett. 112, 177201 (2014).
- [5] A. Ueda, S. Yamada, T. Isono, H. Kamo, A. Nakao, R. Kumai, H. Nakao, Y. Murakami, K. Yamamoto, Y. Nishio, and H. Mori, J. Am. Chem. Soc. 136 (34), 12184 (2014).
- [6] T. Higashino, A. Ueda, J. Yoshida, and H. Mori, Chem. Commun. 53, 3426 (2017).
