Methylation of Epitaxial Graphene on SiC(0001) through Two-Step Chlorination Alkylation Reactions
Md. Z. Hossain and J. Yoshinobu
Chemical modification of graphene with varieties of functionalities is vital for anticipated applications such as sensing, detecting, and composite material systems. However, it is not easy to chemically graft the atoms or molecules onto the graphene surface because of the bonding nature of sp2 C atoms in graphene. Therefore, it is highly desirable to establish a common chemical approach through which the pristine graphene can be modified with varieties of chemical species. In this joint research, we demonstrate the covalent binding of a methyl group on the basal plane of epitaxial graphene on SiC(0001) through the two-step chlorination and alkylation processes using a Grignard reagent (CH3MgBr). The modified surfaces are characterized by scanning tunneling microscopy and spectroscopy, X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy.
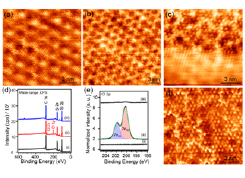
Fig. 1. Typical ambient STM images of (a) the clean epitaxial graphene on the Si-face of the SiC substrate and (b) the same surface after chlorination. (c) Chlorinated and non-chlorinated areas within the same scanning area. (d) Wide range XPS spectra of (i) clean, (ii) chlorinated, and (iii) chlorinated surface treated with CH3MgBr in THF (tetrahydrofuran) for 2 h at 65 °C. hν = 1486 eV. (e) High-resolution Cl 2p XPS spectra of (i) clean, (ii) chlorinated, and (iii) chlorinated surface treated with CH3MgBr in THF. hν = 410 eV. (f) Atomic resolution STM image of the chlorinated surface treated with CH3MgBr. Vtip = 0.5 V; Itunnel = 0.5 nA. [© 2014 American Chemical Society]
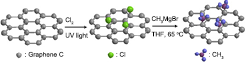
Fig. 2. Schematic of the reaction steps for photochlorination and methylation of epitaxial graphene on SiC using CH3MgBr in THF. [© 2014 American Chemical Society]
Figure 1a is an atomic resolution STM image of monolayer epitaxial graphene (EG) on SiC(0001). The hexagonal network of the graphene lattice is clearly seen over the 6√3× 6√3 substrate structure. The chlorination of EG was done by exposing the surface to Cl2 under UV light in N2 atmosphere. Following the chlorination of graphene, the atomically resolved high-resolution image clearly reveals that the EG contains a large number of small protrusions (Figure 1b). Since the graphene mesh and underlying substrate periodicity are still visible, we ascribe these additional small protrusions to the chemisorbed Cl atoms on EG. Note that the surface morphology shown in Figure 1b is only seen in the monolayer EG region. Figure 1c shows a typical STM image; the hexagonal mesh of the graphene lattice is clearly visible in the lower half of the image, and the upper half contains randomly distributed protrusions ascribed to the chemisorbed Cl. The approximate surface coverage of chemisorbed Cl is estimated to be ~ 8 atom%. Based on the atomic resolution STM and Raman measurements on the different areas of the surface, we conclude that chemisorption of Cl is selective to the monolayer EG on SiC.
The covalent binding of Cl onto the EG is confirmed by the XPS spectra as shown in Figure 1d. The Cl 2p and Cl 2s peaks are observed in the XPS spectrum for chlorinated graphene (Fig. 1d(ii)). The high-resolution Cl 2p spectrum exhibits two components at 200.2 and 201.8 eV (Fig. 1e). When the chlorinated EG is treated with CH3MgBr in THF, the Cl peaks disappear [Figure 1d(iii), e(iii)] and the height of the C 1s peak slightly increased [Figure 1d(iii)] compared to that of the chlorinated surface. The absence of any Mg or Br peaks in the wide range spectra suggests the clean EG modified with only the C containing group. The STM investigations of CH3MgBr treated surfaces indicate that only the monolayer graphene regions contain small protrusions randomly distributed over the surface. The density of small protrusions is similar to that of the respective chlorinated surface. A typical atomically resolved STM image of the CH3MgBr treated surface is shown in Figure 1f. In addition to the small protrusions, we can clearly see the underlying hexagonal mesh of graphene in Fig. 1f. These small protrusions are found to be stable even after annealing the surface at 300 °C in UHV. Since the surface is free from any other contamination as revealed by XPS, we ascribed these small protrusions to the covalently bonded CH3 groups.
The scheme for the present two-step reactions is summarized in Fig. 2, and the detailed analysis of experimental data and discussion is found in reference 1.
References
- [1] Md. Z. Hossain, M. B. A. Razak, H. Noritake, Y. Shiozawa, S. Yoshimoto, K. Mukai, T. Koitaya, J. Yoshinobu, and S. Hosaka, J. Phys. Chem. C 118, 22096 (2014).
