Spin Model of O2-based Magnet in a Nanoporous Metal Complex
Masuda Group
Natural oxygen, the second abundant constituent in the air, is a magnet having spin S = l induced by a couple of πg electrons. Remarkable difference from the magnetism of transition metals is that the geometrical configuration of the O2 molecules and the spin state are closely correlated because the molecular potential between a pair of O2 molecules is strongly dependent on the spin state [1]. To manipulate the oxygen molecule and to artificially synthesize a novel type of O2-based magnet is a challenge in the new field of magnetism.
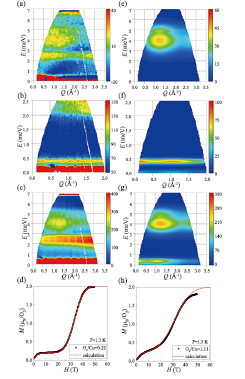
Fig. 1. (a) Inelastic neutron scattering (INS) spectrum for the incident neutron energy of Ei = 7.74 meV in Cu-CHD adsorbing less O2 molecules. (b) INS spectrum for Ei = 3.14 meV in Cu-CHD adsorbing more O2 molecules. (c) INS spectrum for Ei = 7.74 meV in Cu-CHD adsorbing more O2 molecules. (d) Magnetization curve of Cu-CHD adsorbing less O2 molecules. Experimental and calculation results are shown with black dots and red line. (e) Calculated INS spectrum of spin dimer model for Cu-CHD adsorbing less O2 molecules. (f) Calculated INS spectrum of spin trimer model for Cu-CHD adsorbing more O2 molecules in the low energy region. (g) INS spectrum of spin trimer model for Cu-CHD adsorbing more O2 molecules in the high energy region. (h) Magnetization curve of Cu-CHD adsorbing more O2 molecules. Experimental and calculation results are shown with black dots and red line.
We focus our attention on a metal complex having one-dimensional nanopores, Cu-Trans-1,4-Cyclohexanedicarboxylic acid abbreviated as Cu-CHD. The adsorbed O2 molecules form dimer-like structure in the nanopores. Indeed, the magnetic susceptibility of Cu-CHD adsorbing 0.22 mole of O2 per formula unit showed a rapid decrease with the temperature, suggesting a spin-gap system having non-magnetic ground state. It was, however, explained not by S=1 spin dimer but rather by S = 1/2 spin dimer [2]. To explain the unusual bulk property and to identify the precise energy scheme, a spectroscopic experiment is necessary.
In the present study we carried out inelastic neutron scattering (INS) experiments at low temperatures in order to clarify the spin model of the O2-based magnet realized in Cu-CHD [3]. We found that the magnetic excitation of Cu-CHD adsorbing less O2 is explained by a spin-dimers model of which the exchange constants are normally distributed as shown in Figs. 1(a) for experiment and 1(e) for calculation. In contrast, that of Cu-CHD adsorbing more O2 is explained by a spin-trimers model with the distributed exchange constants as shown in Figs. 1(b) and (c) for experiments and Figs. 1(f) and (g) for calculations. It is noted that additional excitation is observed at 0.4 meV in the high concentration O2 sample. The spin model is, thus, tuned by the concentration of O2 in this system. Based on the spin models with the parameters determined by INS experiments and by considering the spin-dependent molecular potential, we quantitatively explained the magnetization curves as shown in Fig. 1(d) for low O2 sample and in Fig. (h) for high O2 sample. The effect of the spin-dependent Van der Waals potential to the anomalous energy scheme is experimentally confirmed.
References
- [1] B. Bussery and P. E. S. Wormer, J. Chem. Phys. 99, 1230 (1993).
- [2] W. Mori, T. C. Kobayashi, J. Kurobe, K. Amaya, Y. Narumi, T. Kumada, K. Kindo, H. Aruga Katori, T. Goto, N. Miura, S. Takamizawa, H. Nakayama, and K. Yamaguchi, Mol. Cryst. Liq. Cryst. 306, 1 (1997).
- [3] M. Soda, S. Takamizawa, S.O. Kawamura, K. Nakajima, and T. Masuda, arXiv:1505.03272.
