Electronic Structure of Carbon-Related Catalysts During Fuel Cell Operation by Soft X-Ray Emission Spectroscopy
H. Niwa, Y. Harada, and M. Oshima
Polymer electrolyte fuel cells (PEFCs) are expected to be promising power sources of high efficiency, low operating temperature, and low pollution for transportation and residential applications. Recently, carbon-based catalysts are getting considerable attention since adequately designed carbon-based catalysts show high ORR activities and are expected to be cathode catalysts alternative to conventional Pt-based catalysts [1,2]. The origin of their high ORR activities should be elucidated to further enhance the activities for commercialization. Previous electronic structure studies on carbon-based catalysts were mostly done at ex situ condition[3]. However, the results were not always conclusive because they did not observe the chemical state of each element under PEFC working condition. To further explore the ORR mechanism of carbon-based cathode catalysts, operando observation of the electronic structure is strongly required.
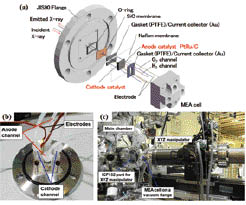
Fig. 1. (a) Schematic view of the MEA cell for operando SXE measurements. (b) Photograph of the MEA cell on a vacuum flange. (c) Photograph of a XYZ manipulator and a main chamber.
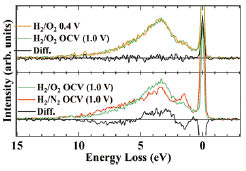
Fig. 2. Smoothed Fe 2p SXE spectra compared with the open circuit voltage (lower panel) and the same gas condition (upper panel). Black solid lines are difference spectra.
Here we report a novel electrochemical cell system developed for operando soft X-ray emission spectroscopy of cathode catalysts for polymer electrolyte fuel cells at BL07LSU of SPring-8[4]. Incorporating a membrane electrode assembly on a vacuum compatible flange (Fig. 1), the system enables direct observation of element-specific electronic structure that changes with gaseous and potential conditions for electrochemical reaction. We have successfully observed the electronic structure of iron in the iron phthalocyanine-based cathode catalyst under various working conditions by the operando soft X-ray emission spectroscopy. At open circuit voltage it is found that an oxidized iron site exists even after reductive high temperature pyrolysis and is active for oxygen adsorption (Fig. 2), which is not expected from ex situ results for powder samples where metallic iron site inactive for ORR dominates.
References
- [1] W. Y. Wong, W. R.W. Daud, A. B. Mohamad, A. A. H. Kadhum, E. H. Majlan, and K. S. Loh, Diam. Relat. Mater. 22, 12 (2012).
- [2] F. Jaouen, E. Proietti, M. Lefèvre, R. Chenitz, J.-P. Dodelet, G. Wu, H. T. Chung, C. M. Johnston, and P. Zelenay, Ene. Environ. Sci. 4, 114 (2011).
- [3] U. I. Kramm, I. Abs-Wurmbach, I. Herrmann-Geppert, J. Radnik, S. Fiechter, and P. Bogdanoff, J. Electrochem. Soc. 158, B69 (2011).
- [4] H. Niwa, H. Kiuchi , J. Miyawaki, Y. Harada, M. Oshima, Y. Nabae, and T. Aoki, Electrochem. Commun. 35, 57 (2013).
