Structure Determination of Silicene on Ag(111) by Low-Energy Electron Diffraction
K. Kawahara, T. Shirasawa, and N. Takagi
Silicene, a 2-dimensional honeycomb lattice of Si, attracts much attention since it is predicted to acquire exotic features such as massless Dirac fermions and high electron mobility [1]. As the spin orbit coupling of Si is 1000 times larger than that of C, silicene is one of the most interesting candidates for 2-dimensional topological insulator [2]. In addition, silicene should match with the current Si based technology. These characteristics make silicene promising in the next generation devices.
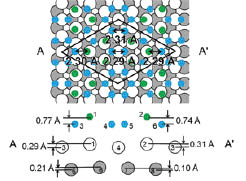
Fig. 1. The top and side views of the best-fit structure of 4×4 silicene. The side view is the cross section along the AA’. Green and blue balls represent Si atoms. The green Si atoms are moved up to the vacuum. White and Gray balls represent Ag atoms in the first and second layer, respectively.
Several groups reported synthesis of silicene on Ag(111) [3-8]. Silicene forms 4×4 superstructure on the Ag(111) surface [4-8]. On a fundamental question ‘Does 4×4 silicene have Dirac fermions?’, the arguments conflict among the research groups [4,7]. The electronic structure is connected with the atomic arrangement. Thus, by determining the atomic position, we can answer the above question. However, the geometric structure of 4×4 silicene has not been established. Several groups proposed the structural models of 4×4 silicene based on the result of scanning tunneling microscopy (STM) [4-8]. The reasonable model is a buckled structural model constructed by the STM and density functional theory (DFT) calculations [4-7]. In contrast, Feng et al. claimed that the corner-hole like features observed in the STM image are assigned to missing Si atoms and proposed a model in which hydrogen atoms terminate the dangling bonds of Si atoms [8]. The discrepancy arises from that the STM image is convolution of the geometric structure together with the electronic density of states. Hence we cannot determine the positions of the individual atoms in the 4x4 silicene only by STM measurements.
Electron diffraction is one of the most reliable and powerful techniques to determine the geometric structure. We have determined the geometric structure of 4×4 silicene by using low-energy electron diffraction [9]. We examined the regularly-buckled silicene, the buckled structure where six Si atoms are displaced and the structural model proposed by Feng et al. The optimized reliability factor (R-factor) of the buckled structure shown in Fig. 1 was 0.17. The optimized R factor of the model proposed by Feng et al. [8] was 0.48. The buckled structural model as Fig. 1 reproduced the experimental results very well. Figure 1 shows the top and cross sectional views of the best-fit structural model for 4×4 silicene. The green Si atoms are displaced perpendicularly to the surface and silicene forms into a buckled structure. The Si-Si bond lengths range from 2.29 to 2.31 Å, which are shorter than that for the bulk diamond structure. Substrate Ag atoms are displaced vertically at the interface. The R-factor is 0.52 for the model without the displacement of Ag, indicating that the displacement of Ag atoms is a key factor to determine the structure of 4×4 silicene. The displacement of Ag also reflects the strong interaction between silicene and Ag substrate, supporting the experimental results showing absence of the Dirac fermions [7]. In addition, the experimentally determined structure of 4×4 silicene matches quite well with that optimized by DFT calculations [4,5]. Thus, the geometric structure of 4×4 silicene on Ag(111) was completely clarified.
References
- [1] S. Cahangirov et al., Phys. Rev. Lett. 102, 236804 (2009). [2] M. Ezawa, Phys. Rev. Lett. 109, 055502 (2012). [3] B. Lalmi et al.,Appl. Phys. Lett. 97, 223109 (2010). [4] P. Vogt et al.,Phys. Rev. Lett. 108, 155501 (2012). [5] C.-L. Lin et al., Appl. Phys. Express 5, 045802 (2012). [6] R. Arafune et al., Surf. Sci. 608, 297 (2013). [7] C.-L. Lin et al., Phys. Rev. Lett. 110, 076801 (2013). [8] B. Feng et al., Nano Lett.12, 3507 (2012). [9] K. Kawahara et al., Surf. Sci. 623, 25 (2014).
