Firefly Bioluminescence Affected by Temperatures and Metal Ions
Akiyama Group
Firefly bioluminescence has attracted great interest among various research fields. The mechanisms of very high quantum yield and condition-sensitive color change of the bioluminescence are long standing issues of basic biochemistry and biophysics, and applications of the bioluminescence such as food hygiene inspection, DNA sequencing, bioimaging, cancer diagnosis, and immunoassay, are under intensive developments and some of them are commercially available. Quantitative study of the bioluminescence is crucially important for both basic research and application, but still very rare [1, 2].

Fig. 1. Picture of temperature-sensitive color change of bioluminescence of North-American Firefly (Photinus pyralis) at pH 6.8 in home-made acrylate tube cell placed on an aluminum sheet.
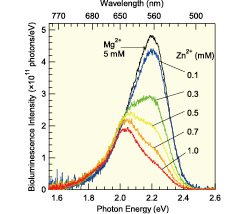
Fig. 2.Time-integrated total photon flux spectra of bioluminescence of North-American Firefly with various zinc-ion densities. Black line shows data with magnesium ion, whose density did not changed the spectra significantly, as a reference.
We measured total-photon flux of firefly bioluminescence in various reaction conditions such as temperature, pH, and bivalent metal density quantitatively. All quantitative spectra were very well reproduced by three Gaussian components, which peaked at 1.9 (red), 2.0 (orange), and 2.2 (green) eV. The positions and widths of the components were insensitive to reaction conditions. Firefly bioluminescence changed its color from yellow-green to yellow, then to orange with increasing temperature as shown in Fig. 1. Quantitative analysis revealed that the color change solely owing to decrease of intensity of the green Gaussian component with increasing temperature. Very similar color change was observed with increasing bivalent metal ion density as shown in Fig. 2. The observed similarity of color change is useful for detailed basic study such as comparisons with quantum-chemistry calculations and the robustness of the luminescence below 2.0 eV can be advantageous as a standard yield for future applications of quantitative measurement of bioluminescence.
References
- [1] Y. Ando et al., Nature Photonics. 2, 44 (2008).
- [2] Y. Wang et al., Photochemistry and Photobiology 87, 846 (2011).
