Oxide Photocatalysts
Lippmaa Group
Perovskite-type titanates have been proposed as potentially efficient photocatalytic energy conversion materials that can absorb visible sunlight and directly transfer the formed photocarriers to liquid water, thereby generating hydrogen gas. Despite the potential for efficient, cheap, and sustainable energy conversion, photocatalytic materials have so far not reached practical solar light collection efficiencies. The main reason appears to be the high recombination rate of generated photocarriers, which means that the energy of the sunlight is mostly spent on generating heat, rather than splitting water. The purpose of this work was to determine the electronic structure of Rh-doped SrTiO3, which is known to be a moderately efficient hydrogen-evolution photocatalyst.
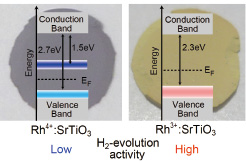
Fig. 1. Photographs of Rh4+:SrTiO3 and Rh3+:SrTiO3 pellets showing the different colors and the Rh in-gap state locations relative to the valence and conduction band edges of SrTiO3. The yellow Rh3+:SrTiO3 is photocatalytically more efficient due to the lack of a mid-gap recombination state.
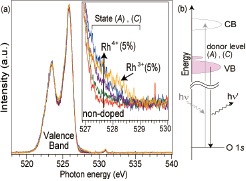
Fig. 2. (a) X-ray emission spectra of various Rh-doped SrTiO3 samples showing the presence of an occupied Rh state close to the top of the valence band of SrTiO3. (b) Illustration of the XES process and the location of the Rh donor levels just above the SrTiO3 valence band edge.
For efficient transfer of photoelectrons from a bulk photocatalyst to water, it is necessary to have a semiconductor with a conduction band located well above the reduction potential of water, while the band gap should be close to 2 eV for the best energy harvesting efficiency. Pure SrTiO3 is a wide-gap semiconductor that satisfies the conduction band alignment requirement with water, but it is transparent for visible light and only absorbs sunlight in the ultraviolet part of the spectrum, above the band gap energy of 3.2 eV. Rhodium doping of the SrTiO3 host semiconductor appears to be quite special, in that it creates deep impurity levels either around the mid-gap region of SrTiO3 or close to the top of the valence band without affecting the location of the conduction band edge. Electrochemical measurements under visible light show an apparent p-type photocathode behavior, which is quite unusual for typically n-type SrTiO3. In this work, we have used a combination of x-ray photoelectron spectroscopy (XPS) at Photon Factory beamline 13A and x-ray absorption (XAS) and emission (XES) spectroscopy at the undulator beamline BL07LSU in SPring-8.
The XPS experiments were used to investigate the violet-to-yellow color change reaction that occurs when Rh:SrTiO3 is reduced, typically during the initial induction period of an electrochemical reaction. SrTiO3 thin films with several different Rh doping levels were grown at various temperatures and ambient oxygen pressures. The Rh 3d XPS profile analysis showed that the color shift is caused by a change in the Rh impurity valence, with Rh4+:SrTiO3 being violet and Rh3+:SrTiO3 yellow [1]. Significant variations were also observed in the Rh content at the catalyst surface due to evaporative loss of Rh at high crystal growth temperatures.
A more detailed analysis of the in-gap energy levels was undertaken by XAS and XES analysis of powder samples. The XAS analysis, which probes unoccupied states, was used to show that a mid-gap unoccupied state appears only in samples containing the Rh4+ valence state, as illustrated in Fig. 1. Reduced Rh3+:SrTiO3 powders did not show a mid-gap state. Both Rh valence states lead to the appearance of an occupied impurity level close to the top of the valence band, as shown by XES spectra in Fig. 2. In addition to an increase of spectral weight with Rh doping just above the valence band top, a shift was observed in the locations of the Rh4+ and Rh3+ impurity levels. A conclusion of the analysis was that the Rh3+:SrTiO3 material shows higher photocatalytic activity due to the lack of a mid-gap unoccupied energy level that would lead to rapid photocarrier recombination. However, the photogenerated charge collection efficiency is limited by the lack of strong hybridization between the deep Rh levels and the O2p character valence band of SrTiO3.
References
- [1] S. Kawasaki, K. Nakatsuji, J. Yoshinobu, F. Komori, R. Takahashi, M. Lippmaa, K. Mase, and A. Kudo, Appl. Phys. Lett. 101, 033910 (2012).
- [2] S. Kawasaki, K. Akagi, K. Nakatsuji, S. Yamamoto, I. Matsuda, Y. Harada, J. Yoshinobu, F. Komori, R. Takahashi, M. Lippmaa, C. Sakai, H. Niwa, M. Oshima, K. Iwashina, and A. Kudo, J. Phys. Chem. C 116, 24445 (2012).
