High-performance Ion Gel
Shibayama Group
Polymer electrolytes using room-temperature ionic liquids (ILs) occupy an important position in the field of solid-state ionics, as well as those containing high-melting temperature salts and/or hard inorganic solid electrolytes. The ILs possess unique properties such as high ionic conductivity, negligible volatility and high thermal stability, therefore, electrochemical research has been performed to explore a new class of electrolytes in device developments. Though many researchers have proposed use of ILs for polymerization solvents and also reported the ionic conductivity of the prepared IL-polymer electrolytes (ion gels), the ionic conductivity decreases at least one order relative to that of the corresponding pure IL. This is because high polymer content is necessary to obtain a free-standing ionic liquid/polymer electrolyte.

Fig. 1. Photographs of Tetra-PEG ion gel (left) and Tetra-PEG hydrogel (right) showing the difference in the stability against heat. Both gels were placed on a hot plate at 100 °C. By time went from 0 min (a) to 120 min (b), the hydrogel was completely dried, while the ion gel remain as it had been.
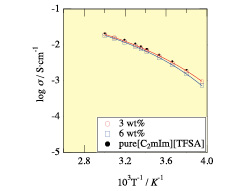
Fig. 2. Temperature dependence of ionic conductivity for 3 (red circles with line) and 6 wt% (blue squares with line) Tetra-PEG ion gels containing [C2mIm+][TFSA-] (red and blue, respectively), together with that for pure IL (black dots). The solid lines show the fitting curve with VFT equation.
We succeeded in preparation of ion gels consisting of imidazolium-based ionic liquids and Tetra-PEG network [1]. Here, Tetra-PEG denotes tetra-arm poly(ethylene glycol). Hereafter, we call the obtained polymer gel “Tetra-PEG ion gel”. The Tetra-PEG ion gel was prepared by mixing two Tetra-PEG macromers in ILs at room temperature. The polymer concentrations were 3 and 6 wt%. The preparation of Tetra-PEG gel is reported elsewhere [2]. The ILs examined were 1-ethyl-3-metylimidazolium bis(trifluoromethanesulfonyl)amide, [C2mIm+][TFSA-] and 1-ethyl-3-metylimidazolium bis(fluorosulfonyl)amide, [C2mIm+][FSA-]. Fig. 1 shows time-course of photographs of Tetra-PEG ion gel (left) and hydrogels (right) placed on an electric hot plate (a) 0 min and (b) after 120 min. The surface temperature of the hot plate was 100 °C. As shown in the figure, Tetra-PEG ion gel did not change at all, while Tetra-PEG hydrogel was dried completely within 120 min. Fig. 2 shows the ionic conductivities, σ, for 3 and 6 wt% Tetra-PEG ion gels. The obtained values of σ are very close to that of pure solvent, i.e., [C2mIm+][TFSA-]. At room temperature, the σ values are 8.5 and 7.7 × 10-3 S cm-1 for 50 and 100 mg/ml Tetra-PEG ion gels, respectively, which are much higher than the values already reported for conventional polymer electrolytes. As seen in this figure, the σ value slightly decreases with increasing Tetra-PEG concentration. However, the σ value at higher concentrations remained 85% of pure IL (9.1 × 10-3 Scm-1). The experimental data were well represented by the Vogel-Tamman-Fulcher (VTF) equation for conductivity for such electrolytic materials, which is shown by solid lines in Figure 2. The VFT equation is given by σ = σ0exp[-B/(T – T0)] where σ0, B and T0 are adjustable parameters. The mechanical properties were examined by stretching as well as compression measurements and it was found that the ion gels are strong enough to be used as a free-standing electrolyte. Uniformity of the network structure of Tetra-PEG ion gels was confirmed by small-angle neutron scattering.
A free-standing ion gel with high strength and toughness was obtained by directly cross-end-coupling of two Tetra-PEG macromer (3.2 and 6.4 wt%) in room temperature ionic liquid. The ionic conductivity is practically equal to that of pure IL. The mechanical properties of Tetra-PEG ion gels are comparable to those of high-strength Tetra-PEG hydrogels [3].
References
- [1] K. Fujii, H. Asai, T. Ueki, T. Sakai, S. Imaizumi, U. Chung, M. Watanabe, and M. Shibayama, Soft Matter 8 (2012).
- [2] T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama, and U. I. Chung, Macromolecules 41 (2008).
- [3] H. Asai, K. Fujii, T. Ueki, T. Sakai, U. Chung, M. Watanabe, Y. S. Han, T. H. Kim, and M. Shibayama, Macromolecules 45 (2012).
