Identification of the Key Interactions in Structural Transition Pathway of FtsZ from Staphylococcus Aureus
Y. Shigeta, R. Harada, and H. Matsumura
The bacterial cell division protein FtsZ forms a ring-shaped filament (Z-ring) inside the cell membrane in the presence of GTP, and dynamically repeats division and dissolution owing to treadmilling coupled with the GTPase activity to cause invagination of the cell membrane. Although this invagination is thought to be caused by shrinking of the Z-ring, its molecular mechanism is still unknown. The crystal structure of GDP-bound Staphylococcus aureus FtsZ (SaFtsZ-GDP complex) was experimentally determined by X-ray structural analysis. Surprisingly, there are two different three-dimensional structures in the same crystal, which correspond to GTP- and GDP-bound forms (or tense (T) and relaxed (R) states) in the other species. Visualization of the two states in the same species enables us to investigate the structural transition pathway between the T and R states, which might be related to the constriction of the Z-ring. Generally, in order to reproduce large-scale structural transitions related to biological functions in silico, extremely long-time molecular dynamics (MD) simulation is required. To avoid this problem, it is necessary to apply some structure sampling method in order to reproduce important structural changes efficiently as quickly as possible. In this research, we apply parallel cascade selection MD (PaCS-MD) simulation developed by us [1, 2] for searching structural transition pathways between the T and R states [3]. After obtaining the transition pathway we have evaluated free energy landscape (FEL) and find transition state (TS) structure (see Fig. 1). From the FEL, the free energy difference and barrier between the two states are merely kBT and 4kBT, respectively, indicating the monomeric SaFtsZ-GDP complex exists in equilibrium. During the transition, it is found that the main chain carbonyl group of Arg29, whose side chain moves significantly between the T and R states, tentatively forms a hydrogen bond with Arg191 nearby in the TS. From these analysis, it is found that Arg29 is a key amino acid residue for the function of FtsZ, and a subsequent mutagenesis work also support the hypothesis.
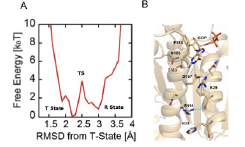
Fig. 1. (A)Free energy landscape of SaFtsZ, which connects T- and R-state. (B) Transition state structure during T- and R-state transition around GDP binding site.
References
- [1] R. Harada, and A. Kitao, J. Chem. Phys. 139, 035103 (2013).
- [2] R. Harada, Y. Takano, T. Baba, and Y. Shigeta, Phys. Chem. Chem. Phys. (feature article) 17, 6155 (2015).
- [3] J. Fujita, R. Harada, Y. Maeda, Y. Saito, E. Mizohata, T. Inoue, Y. Shigeta, H. Matsumura, J. Struct. Biol. 198, 65 (2017).
