Interface Hydrogen Bonds at the HeteroJunction between Proton-Donating and Proton-Accepting Self-Assembled Monolayers
I. Matsuda, Mori, amd Yoshinobu Groups
Hydrogen bondings have been significant in chemical and biological systems. It has been known to fix molecular configurations and it is also flexible for breaking and making bonds between molecules at room temperature. Additionally, a hydrogen bond also effectively assists the proton (H+) transfer between the donor and the acceptor sites along the bond. The transfer of one whole H+ ion substantially changes charge distributions between the molecules. Based on this electron-proton concerting effect, highly conductive pure organic molecular crystals have been realized at the partially deprotonated molecular crystals of catechol-fused ethylenedithiotetrathiafulvalene (H2Cat-EDT-TTF). These facts motivate us to design a possible control of the film conductivity depending of the proton position between H+ donor and acceptor layers, which leads to a new concept for driving electronic device. As the first step of this ambitious goal, we created a hydrogen-bonding heterogeneous interface consisting of a bilayer of the H+-donating and –accepting molecules on a substrate (Fig. 1).
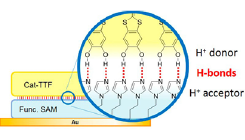
Fig. 1. Schematic drawing of hydrogen bonds at the interface between Cat-TTF and functional SAM (Im-SAM) on Au(111).
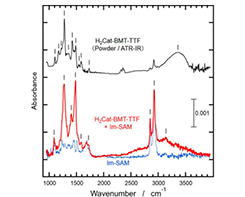
Fig. 2. Infrared (IR) reflection absorption spectra for H2Cat-BMT-TTF adsorbed on Im-SAM (red) and for Im-SAM (blue), in comparisons with the IR spectrum for the H2Cat-BMT-TTF powder (black).
For the H+-acceptor molecular layer, we adopted an imidazole-terminated alkanethiolate self-assembled monolayer (Im-SAM) prepared on the Au(111) substrate. The imidazole molecule can accept H+ at the imino N site and stabilize it as an imidazolium cation. At the saturation coverage of alkanethiolate-based SAMs, the molecules are in a closely packed standing-up form and the end group tends toward the open side on the SAM. The molecular orbitals and chemical properties of the functional end groups are hardly perturbed from Au substrates due to the sufficient thickness of the closely packed alkylene chains in the SAMs. Thus, the imino N atom on Im-SAMs is expected to make hydrogen bonds as H+ acceptor sites with H+-donating molecules.
For the counterpart, the H+-donor molecular layer, we focused on the adsorption of the catechol-fused bis(methyl-thio)tetrathiafulvalene (H2Cat-BMT-TTF) molecules on the Im-SAM film. The hydroxyl groups at the catechol moiety in H2Cat-BMT-TTF should act as the H+-donating group, by analogy to the conductive H2Cat-EDT-TTF complexes, in which it has been elucidated that the TTF moiety is electron-donating and the hydroxyl groups in the catechol moiety accept the electron by pushing out H+.
We confirm the formation of a hydrogen-bonding heterogeneous bilayer that consists of the H2Cat-BMT-TTF layer and the Im-SAM film, systematically elucidated by AFM, X-ray photoelectron spectroscopy, infrared absorption spectroscopy, and density functional theory calculations. Figure 2 shows one of the evidence showing clear infrared spectral signals of the bilayer system. The broad feature at frequency of 2500-3500cm-1 indicates strong hydrogen bonds at the heterointerface. These results provide new insight for designing electron-proton functions in adsorption systems.
References
- [1] H. S. Kato, S. Yoshimoto, A. Ueda, S. Yamamoto, Y. Kanematsu, M. Tachikawa, H. Mori, J. Yoshinobu, and I. Matsuda, Langmuir 34, 2189 (2018).
