Stability of Titania-Terminated Surfaces in Water
Lippmaa Group
Photoelectrochemical water splitting can be used to collect solar energy and generate hydrogen gas from water. While this process offers an attractive pathway to a clean energy supply, the low energy conversion efficiency presents several challenging unsolved materials science problems. One of the requirements for a suitable photoelectrode material is long-term stability in water during an electrochemical reaction. Titanium oxides such TiO2 and SrTiO3 are two examples of water-stable oxide semiconductors that have been widely studied in this context. However, even for well-ordered single crystal surfaces, it is difficult to determine to what extent the electrochemical reaction affects the composition or structure of the photoelectrode surface.
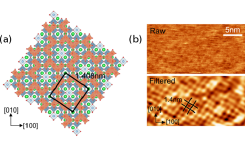
Fig. 1. (a) Surface model of the SrTiO3 (√13× √13) surface. Top layer Ti is shown in orange, second layer Ti in blue, Sr in green and O in red. (b) Raw and filtered FM-AFM images of the reconstructed surface measured in water.
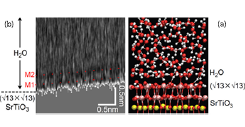
Fig. 2. (a) MD simulation of the water structure in the hydration layer above the SrTiO3 (√13×√13) surface. Sr is yellow, Ti is pink, O is red, and H is gray. (b) FM-AFM frequency shift map as a function of lateral position and distance from the surface. The local water density minima are marked M1 and M2.
The purpose of this work was to analyze the oxide electrode surface stability and the oxide-water interface structure by numerical simulations and the measurement of surface hydrophilicity, atomic-scale surface morphology, and the local water density. SrTiO3 (001) single crystals with a (√13×√13)-R33.7° surface reconstruction were used for the experimental work. This surface is terminated by a partial TiO2 double layer (Fig. 1a) that is chemically stable in water and the presence of the reconstruction pattern can be observed either by scanning probe or electron diffraction. The reconstructed (√13×√13) surface can thus function as a stability marker when a crystal is subjected to various surface treatments. If the reconstruction remains detectable after a process step, we can be sure that the surface has not been structurally altered or etched. For a typical (1 × 1) SrTiO3 surface, it would be much harder to detect structural alterations in the topmost unit cell of the crystal. A high-resolution atomic force microscope (AFM) image of the (√13×√13) surface measured in water is shown in Fig. 1b. The presence of the expected periodicity shows that the surface structure is not damaged when exposed to water.
The hydration layer structure on the (√13×√13) surface was simulated by density functional theory molecular dynamics to evaluate the surface stability of various crystal surface terminations. The simulation result shown in Fig. 2a indicates that a layered water molecule configuration may be expected to exist at the surface. The presence of a structured hydration layer was verified by frequency-modulation AFM in an electrolyte solution (Fig. 2b). The AFM tip was scanned laterally over the crystal surface a height scan was recorded at each lateral point, forming a depth map of water density at the surface. Two dark bands can be seen in the AFM frequency shift map in Fig. 2b, marked with M1 and M2. The density variation matches the simulation result, indicating that a stable hydration layer forms on the reconstructed SrTiO3 surface and no chemical etching, even on a single atomic layer scale, could be observed. The presence of the reconstructed surface was verified after water exposure by high-energy electron diffraction.
Titania surfaces are known to exhibit photoinduced hydrophilicity, where the water contact angle drops dramatically upon ultraviolet illumination of a crystal surface. The hydrophilicity of the reconstructed SrTiO3 surface was measured immediately after surface preparation in a vacuum chamber, showing that the surface is intrinsically superhydrophilic. A gradual contact angle increase occurs during several minutes of air exposure, but the superhydrophilic surface character can be regained by vacuum heating without the loss of the reconstructed surface structure. This indicates that the photoinduced hydrophilicity of the surface is related to surface contamination, rather than photoinduced structural degradation of the crystal. The work thus shows that SrTiO3 surfaces terminated with a double TiO2 layer are highly stable in water, even under ultraviolet exposure and can thus be used in photoelectrochemical water splitting cells that require long term electrode stability.
References
- [1] S. Kawasaki, E. Holmström, R. Takahashi, P. Spijker, A. S. Foster, H. Onishi, and M. Lippmaa, J. Phys. Chem. C 121, 2268 (2017).
