Dynamics of Hydrogen Atoms in Nanocrystalline Palladium Hydride
Yamamuro Group
Palladium hydride (PdHx) is the most popular metal hydride which has been investigated by many physicists and chemists. It has been remarked also from industrial points of view, e.g., hydrogen storage, filters, sensors, catalysts, etc. The physical and chemical properties of nanometer-sized materials have also been actively studied since they are often different from bulk properties owing to their size and/or surface effects. We have performed calorimetric [1] and neutron diffraction [2] studies on nanocrystalline PdHx and PdDx. In bulk samples, H atoms are located at the octahedral (O) sites in an fcc lattice (see the inset of Fig. 1). We found that H atoms occupy not only the O sites but also the tetrahedral (T) sites in the subsurface region of nanoparticles.
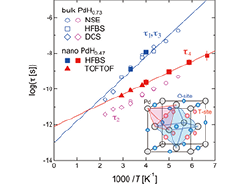
Fig. 1. Arrhenius plots of the relaxation times for the hydrogen motions in nanocrystalline PdH0.47 and bulk PdH0.73. All of the plotted data are the values at Q = 0.8 Å−1. The solid lines represent the results of the fits assuming the Arrhenius law. The inset shows the locations of interstitial hydrogen atoms in an fcc Pd lattice; octahedral (O) sites (1/2,1/2,1/2) and tetrahedral (T) sites (1/4,1/4,1/4).
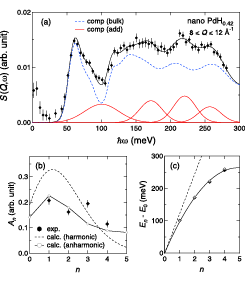
Fig. 2. (a) Inelastic neutron scattering spectrum for nanocrystalline PdH0.42 at 10 K. (b) Intensity and (c) excitation energy against a quantum number n.
Recently, we conducted the quasielastic neutron scattering (QENS) and inelastic neutron scattering (INS) works on bulk and nanocrystalline PdHx [3,4]. These neutron scattering methods are very powerful to explore the dynamics of hydrogen atoms owing to a large incoherent scattering cross section for a H atom. The QENS experiments were performed on HFBS, DCS and NSE at NCNR, NIST (USA) and TOFTOF at FRM II, TUM (Germany), and the INS experiment on 4SEASONS at MLF, J-PARC (Japan).
Figure 1 shows the temperature dependence of the relaxation times of the hydrogen motions in bulk and nanocrystalline PdHx [3]. These values were obtained by fitting the QENS spectra to Lorentz functions (τ is an inverse of half width at half maximum) or exponential functions (for NSE data). In the bulk sample, τ1 and τ2 are associated with the O-O jump motions among the ground states and among the first excited states, respectively. In the nanocrystalline sample, τ3, which is comparable with τ1, may be due to the O-O jumps in the interior region of the nanocrystals. On the other hand, τ4, which is a novel relaxation, may be due to the T-T jumps at around the subsurface of the nanocrystals taking the previous structural information [2] into consideration.
Figure 2(a) presents the INS spectrum of nanocrystalline PdH0.42 [4]. The spectrum was fitted with the combination of the spectrum of the bulk PdH0.73 (dashed curve) and the additional excitations (solid curves). The additional component was represented by multiple Gaussians taking peak positions, intensities and widths as fitting parameters. The estimated intensities and averaged excitation energies are displayed in Fig. 2(b) and (c). Dashed curves represent the expectation from the quantum harmonic oscillator model (QHO) with hν0 = 122 meV. Obviously, the QHO model does not reproduce the experimental results; the data were roughly reproduced by a highly anharmonic trumpet-like potential. The additional excitations are attributed to the H vibrations at the T sites in the subsurface region. Thus, by using neutron scattering techniques, we have obtained structural, diffusion, and vibrational data which are consistent with each other.
References
- [1] H. Akiba et al., Phys Rev. B 92, 064202 (2015).
- [2] H. Akiba et al., J. Am. Chem. Soc. 138, 10238 (2016).
- [3] M. Kofu et al., Phys Rev. B 94, 064303 (2016).
- [4] M. Kofu et al., submitted to Phys Rev. B.
