Real-Time Operando Observation of Reaction Processes of CO2 on Cu(997) by Near-ambient Pressure X-ray Photoelectron Spectroscopy
Yoshinobu and I. Matsuda Groups
Activation of CO2 is an important topic in the efficient use of CO2 as a chemical feedstock. Methanol synthesis from CO2 and H2 on a Cu/ZnO catalyst has been widely studied. CO2 is chemically inert, and thus the interaction of the molecule with metallic Cu surfaces plays an essential role for molecular activation. On metallic copper, very low reactivity of CO2 on low-index surfaces was reported under ultrahigh vacuum conditions. In contrast to the flat surfaces, the dissociation of CO2 into CO was reported on vicinal Cu surfaces previously. These results indicate that defect sites, such as step and kink, may be important for the CO2 activation. However, the reaction condition in UHV is far from a real catalytic reaction, which is normally operated at higher temperature and nearly/above atmospheric pressure. Such differences in pressure and temperature may lead to distinct reactivity under ambient conditions from UHV, reflecting thermodynamic and kinetic effects. Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) is a powerful tool for investigating electronic structures and chemical states of adsorbates and a substrate quantitatively under reactant-gas pressure.
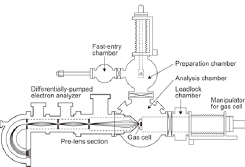
Fig. 1. Schematic of the operando AP-XPS system at SPring-8 BL07LSU
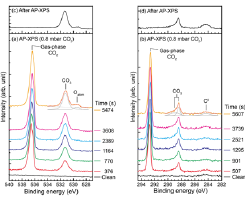
Fig. 2. A series of (a) O 1s and (b) C 1s AP-XPS spectra of Cu(997) at 340 K under CO2 pressure of 0.8 mbar as a function of elapsed time together with the assignment of each peak. Only selected spectra from the whole series are shown in the figure. The photon energy was 630 eV. The CO2 gas was introduced in the gas cell at t = 0 s. The fitting results for the spectra at t = 5474 s (O 1s) and t = 5607 s (C 1s) are also shown in the figure. (c) O 1s and (d) C 1s XPS spectra after the AP-XPS measurements shown in (a) and (b). The gas cell was evacuated to UHV (~10-9 mbar), and then the XPS measurements were performed.
Here, the reaction of CO2 on the vicinal Cu(997) surface at 340 K under CO2 gas pressure of 0.8 mbar was investigated by ambient pressure X-ray photoelectron spectroscopy (AP-XPS). We found that initially carbonate (CO3) was produced on the surface through the reaction of CO2 with oxygen formed from CO2 dissociation; the amount of adsorbed CO3 was increased and saturated as time elapsed and after saturation of adsorbed CO3, atomic oxygen appeared on the surface, indicating that CO2 dissociation into CO and O continued to take place. The estimated saturation coverage of CO3 from XPS is rather small (0.05 ML).
The experiments were performed using a newly developed AP-XPS apparatus at the soft X-ray undulator beamline BL07LSU of SPring-8. Figure 1 shows a schematic of the present operando AP-XPS system at SPring-8 BL07LSU. The AP-XPS system consists of four interconnected UHV chambers; an analysis chamber, a preparation chamber, a load-lock chamber, and a fast-entry chamber. The analysis chamber is used for XPS measurements both in UHV and at ambient conditions. The preparation chamber is equipped with an ion source and low energy electron diffraction optics. The load-lock chamber and the fast-entry chamber allow the introduction of samples from the air into the UHV system.
Figures 2(a) and (b) show series of O 1s and C 1s AP-XPS spectra of the Cu(997) surface at 340 K under CO2 pressure of 0.8 mbar as a function of elapsed time. Gas-phase CO2 peaks were initially observed at 536.6 eV in an O 1s XPS spectrum at t = 376 s, and at 292.8 eV in a C 1s XPS spectrum at t = 507 s. These gas-phase peaks were shifted to 536.4 eV and 292.6 eV, respectively, after a lapse of about 5500s. At t = 376 s, a peak of adsorbate was observed at 531.3 eV in the O 1s region. Three peaks of adsorbates were observed in the C 1s spectrum (t = 507 s) at 288.4 eV with a broader shoulder peak at higher binding energy (289.0 eV), and at 284.4 eV (C0), which is assigned to neutral carbon species such as carbon atoms and hydrocarbons. As discussed later, the other adsorbate peaks at 531.3, 289.0 and 288.4 eV are assigned to carbonate (CO3). The amount of CO3 was saturated at t ~ 2000 s, and then a new peak at 529.5 eV appeared in O 1s spectra. This peak is attributed to atomic oxygen. Figures 2(c) and (d) show O 1s and C 1s spectra measured under UHV (~10-9 mbar) after a series of AP-XPS measurements. The adsorbates formed in the presence of 0.8 mbar CO2 were stable at 340 K and remained on the surface even after evacuating the gas cell to UHV. The result indicates that the decomposition of CO3 does not occur at 340 K.
The composition ratio between oxygen and carbon at the saturation region (t > 2000 s) was calculated from the area intensity of the O 1s peak at 531.3 eV and the sum of the intensities of the two C 1s peaks at 289.0 and 288.4 eV. In the estimation, the O 1s and C 1s intensities of the adsorbate peaks were normalized by those of the gas-phase CO2 peaks in these spectra to cancel out differences in the analyzer transmission function and the core-election ionized cross section between O 1s and C 1s. The O/C ratio was estimated to be 3.1 ± 0.1. Similar experiments were done using a photon energy (hν) of 740 eV to check the effect of photoelectron diffraction on the estimation of the O/C ratio. The estimated ratio at hν = 740 eV is 3.2 which is within the error in the case of hν = 630 eV. If the observed two peaks in C 1s are due to both CO3 and chemisorbed CO2, the O/C ratio should be in the range from 2 to 3. Thus, the peaks at 531.3, 289.0 and 288.4 eV are assigned to CO3.
The produced CO3 is stable at 340 K even after evacuation to UHV as shown in Figs. 2(c) and (d). The gas-phase peaks in AP-XPS spectra (Figs. 2a and b) are shifted to lower binding energies with increasing the CO3 coverage, indicating the increase of the work function by the CO3 adsorption. Therefore, adsorbed CO3 is negatively charged by charge transfer from the Cu substrate. The present study clearly shows a facile formation of CO3 on the stepped Cu surface, and CO3 may be a key intermediate in CO2 activation.
References
- [1] T. Koitaya et al., Top. Catal. 59, 526 (2016).
