A New Structure Family of Oxide-Ion Conductor NdBaInO4
M. Yashima and K. Fujii
Oxide-ion conducting materials such as pure oxide-ion conductors and mixed oxide ion-electronic conductors have a wide variety of applications in fuel cells, oxygen separation membranes and gas sensors. Since the oxide-ion conductivity is strongly dependent on the crystal structure, the discovery of new oxide-ion conductor belonging to a new structure family may open a new window for further innovative developments in the applications of oxide-ion conductors. Here, we report a new structure family of oxide-ion conductor NdBaInO4 (Fig. 1) [1, 2].
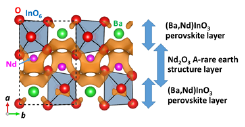
Fig. 1. Refined crystal structure and orange isosurface of the difference bond valence sum distribution for an oxide ion (O2-) of NdBaInO4. The structure consists of (i) Nd2O3 A-rare earth structure- and (ii) (Ba,Nd)InO3 perovskite-layers. The difference bond valence sum map suggests that the oxide-ion conduction occurs in the Nd2O3 layer.
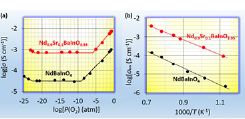
Fig. 2. (a) Oxygen partial pressure dependence of total electrical conductivity at 858 oC of NdBaInO4 (Black) and Nd0.9Sr0.1BaInO3.95 (Red). (b) Arrhenius plots of oxide-ion conductivity of NdBaInO4 (Black) and Nd0.9Sr0.1BaInO3.95 (Red).
To design a new layered perovskite-related structure, we have examined various chemical compositions of AA'BO4 where A and A' are larger cations and B is smaller cation. After examining a number of chemical compositions, we discovered a new structure family of oxide-ion conducting materials NdBaInO4 [1]. The Nd, Ba and In were chosen as cations, because (i) the different sizes of Nd and Ba can lead to the Ba/Nd cation ordering and (ii) the BaInO3 perovskite layer can form from the view point of the sizes of Ba and In cations. Nd1-xSrxBaInO4-x/2 samples were prepared by solid-state reactions at 1400 oC using BaCO3, SrCO3, In2O3 and Nd2O3 powders. NdBaInO4 and Nd0.9Sr0.1BaInO3.95 exhibit oxide-ion conduction as shown in Figure 2. NdBaInO4 and Nd0.9Sr0.1BaInO3.95 samples are a single monoclinic phase with a new crystal structure as described below. Thus, a new structure family of oxide-ion conducting material NdBaInO4 was discovered in this study. The oxide-ion conductivity of NdBaInO4 is improved by Sr substitution at the Nd site (Fig. 2).
Crystal structure of NdBaInO4 was investigated by neutron and synchrotron X-ray powder diffractometry and ab initio electronic calculations. We carried out the ab initio crystal structure analysis using the X-ray powder diffraction data. Neutron diffraction enables the precise determination of positional parameters of oxygen atoms. The space group was found to be monoclinic P21/c. The validity of the crystal structure of NdBaInO4 (Figure 1) was confirmed by Rietveld refinements of synchrotron X-ray powder diffraction data measured at (i) SPring-8 and at (ii) PF, by Rietveld analysis of neutron diffraction data measured at (iii) J-PARC at (iv) ANSTO, and at (v) KAERI, (vi) by bond valence sums (BVS) of Nd, Ba and In atoms, and (vii) by the structural optimization based on the density functional theory (DFT) calculations. The refined crystal structure of NdBaInO4 consists of (i) Nd2O3 A-rare earth structure- and (ii) (Ba,Nd)InO3 perovskite-layers (Fig. 1), which indicates a new A/A' (Nd/Ba) cation ordered perovskite-related layered structure. An outstanding and unique feature of this new structure is that the edge of the InO6 octahedron faces the A2O3 (Nd2O3) layer. The oxide-ion diffusion path of NdBaInO4 was studied by the bond valence method (Fig. 1). The oxide ions can diffuse two-dimensionally in the A2O3 (Nd2O3) layer.
References
- [1] K. Fujii, Y. Esaki, K. Omoto, M. Yashima, A. Hoshikawa, T. Ishigaki, and H. R. Hester, Chem. Mater. 26, 2488 (2014).
- [2] K. Fujii, M. Shiraiwa, Y. Esaki, M. Yashima, S. J. Kim, and S. Lee, J. Mater. Chem. A 3, 11985 (2015).
