Silver Oxide Clathrate Thin Films
Y. Matsumoto, R. Takahashi, and M. Lippmaa
We have studied the possibility of growing epitaxial films of silver clathrates on single-crystal oxide substrates by photoelectrochemical deposition. The clathrates chosen for this study were Ag7O8NO3 and Ag7O8HSO4. Both materials consist of a cubic lattice of Ag6O8 cages that enclose either NO3 or HSO4 anions, as illustrated in Fig. 1(a). Silver clathrates have various interesting properties; Ag7O8NO3, for example, becomes superconducting at 1 K. Our purpose was to develop a technique for fabricating epitaxial clathrates with well-defined orientation on a single-crystal substrate. Silver clathrates contain Ag ions in several valence states, including Ag+, Ag2+, and Ag3+, which means that strongly oxidizing synthesis conditions are needed. One possibility is to use photoelectrochemical deposition on a metallic substrate under a suitable substrate bias in either AgNO3 or Ag2SO4 solution. Growth experiments on 0.5 wt% Nb-doped TiO2 substrates showed that the best selectivity for clathrate formation can be obtained at a bias of +0.2V vs. Ag. The necessary photoexcitation was provided by a high-pressure mercury lamp at a flux of 28 mW/cm2. X-ray diffraction analysis showed that the best orientation control could be achieved on TiO2 (110) substrates, yielding micrometer-scale epitaxial platelets, shown in a scanning electron microscope image in Fig. 1(b). It is clear from the image that the platelets tend to assume a hexagonal shape, characteristic of (111)-oriented growth. The growth habit and epitaxial relationship were further studied by x-ray diffraction and epitaxial relationships were found.
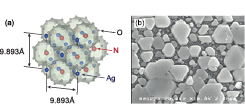
Fig. 1. (a) Structure of Ag7O8NO3 silver clathrate crystals that consist of a cubic lattice of Ag6O8 cages that enclose NO3 anions. (b) SEM image of epitaxial (111)-oriented Ag7O8NO3 platelets photodeposited on a TiO2(110) substrate surface in a AgNO3 solution at an electrode potential of +0.3V vs Ag.
The reason for the preferential formation of epitaxial clathrate crystals on TiO2 (110) surfaces was analyzed in terms of lattice matching between the clathrate Ag6O8 cage pseudo lattice and the TiO2 surface. The (110)-oriented TiO2 surface has a tetragonal structure where the spacing between adjacent bridging oxygen rows is 6.495 Å. This distance matches reasonably well the clathrate cage spacings of 6.056 Å, when the clathrate lattice is viewed along the (111) direction. It appears that despite the 6% mismatch, an epitaxial relationship is formed during platelet growth. The growth mechanism is thus somewhat similar to the formation of C60 films on crystalline substrates, although the growth of a hexagonal (111)-oriented lattice on a tetragonal surface is unusual.
Various different anions can be integrated in the clathrate lattice. We compared the growth habit and growth rates of silver clathrates containing the HNO3 and HSO4 anions and found that while the growth rate of Ag7O8HSO4 was much higher than for Ag7O8NO3, the epitaxial relationship was weaker. Since the lattice parameter of the clathrates is predominantly set by the size of the Ag6O8 cages, it appears that the formation of large epitaxial platelets is limited by other factors, with variable anion incorporation kinetics in the Ag6O8 cages being the most likely reason. This work showed that epitaxial growth of inorganic cage compounds is possible when commensurate matching between the cage pseudo lattice and a single-crystal substrate occurs.
References
- [1] R. Tanaka, R. Takahashi, S. Takata, M. Lippmaa, and Y. Matsumoto, CrystEngComm 17, 3701 (2015).
