Neutron Diffraction of Nano-crystalline PdD
Yamamuro Group
Palladium hydride (PdHx) is the most popular metal hydride which has been investigated by many physicists and chemists. It has been remarked also from industrial points of view, e.g., hydrogen storage, filters, sensors, catalysts, etc. On the adsorption process, a hydrogen molecule (H2) dissociates into two hydrogen atoms (2H). In the H concentration region higher than x = 0.6, the hydride crystal takes the β phase with an fcc (NaCl-type) structure as show in Fig. 1(a). It is known that the H atoms are located at the octahedral (O) sites (1/2, 1/2, 1/2).
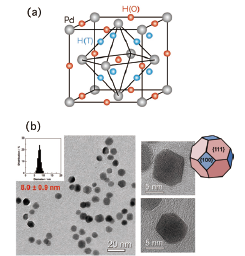
Fig. 1. (a) Structure of the β phase of Pd hydrides. The red and blue spheres show the possible octahedral and tetrahedral sites for hydrogen atoms, respectively. The gray spheres show the Pd fcc lattice. (b) Photographs of transmission electron microscope (TEM) for nano-crystalline Pd. The size distribution of the nano-particles and the schematic drawing of an edge-cut octahedron are also shown.
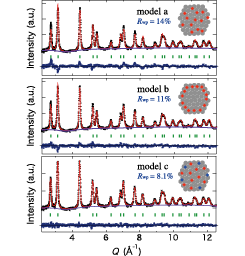
Fig. 2. Neutron powder diffraction pattern of nano-crystalline PdD0.363 at 300 K and 0.1 MPa. The Rietveld analysis was performed assuming that D atoms are located at (a) O sites only, (b) O sites around the surface and subsurface, (c) both O and T sites.
The physical and chemical properties of nanometer-sized materials are of interest since they are often different from bulk properties. As for the nano-particles of palladium hydrides, there are drastic changes of H2 pressure-composition phase diagram [1], structures [2,3], etc. Inelastic neutron scattering (INS) [4] and quasielastic neutron scattering (QENS) [5] works have also been performed though the size of particles were relatively large (20-30 nm) and there were adhesions between particles. In this work, to clarify the structure including hydrogen atoms, we have performed in situ neutron powder diffraction experiments for high-quality nano-crystalline Pd as shown in Fig. 1(b). The nano-particles form an edge-cut octahedron enclosed by {111} and {100} facets, and its diameter is 8.0±0.9 nm.
Figure 2 shows the diffraction pattern of nano-crystalline PdD0.363 measured at 300 K on a total scattering diffractometer NOVA at J-PARC. The amount of the absorbed deuterium (D) atoms into nano-crystalline Pd is precisely determined by the hydrogen gas control system installed on NOVA. The data shown in Fig. 2 are obtained after subtracting the large contribution of incoherent scattering from a protection polymer polyvinylpyrrolidone (PVP) which is used to avoid the adhesion between the nano-particles. This is the first neutron diffraction data for nano-crystalline metal hydrides. The crystal structure of nano-crystalline PdDx has been refined by the Rietveld method as shown by the red curves of Fig. 2. We have examined the following three structural models: (model a) the O sites are occupied by the D atoms homogeneously, (model b) the O sites at the surface and subsurface (a few layers near the surface) are occupied, (model c) both the O sites and tetrahedral (T) sites (1/4,1/4,1/4) are occupied homogeneously. The best fit result was obtained with the model c. The obtained fitting parameters are as follows: lattice constant a = 4.010(4) Å, isotropic atomic displacements BPd = 0.322(8) Å2, BD(O) = 1.93(8) Å2, BD(T) = 14.8(9) Å2, occupancies of the O and T sites gD(O) = 0.249 and gD(T) = 0.057 under the constraint x = gD(O) + 2gD(T) = 0.363.
Thus, our Rietveld analysis has revealed that D atoms occupy not only the O sites but also the T sites in nano-crystalline Pd. We guess that the T site occupation is due to the change in potential energy caused by the surface and/or distortion effects of nano-particles. The present results will give an important clue to the drastic change of the H atom dynamics in Pd nano-particles revealed in our recent preliminary neutron scattering work.
References
- [1] M. Yamauchi et al., J. Phys. Chem. C 112, 3294 (2008).
- [2] M. Suleiman et al., J. Alloys and Compounds 644, 356 (2003).
- [3] J. A. Eastman et al., Phys. Rev. B 48, 84 (1993).
- [4] U. Stuhr et al., J. Phys.: Condens. Matter 7, 219 (1995).
- [5] S. Janßen et al., NanoStruct. Mater. 9, 579 (1997).
