Structure and Dynamics of Domains in Imidazolium-based Ionic Liquids
Yamamuro Group
Ionic liquids (ILs) are ionic compounds whose melting temperatures are around or lower than room temperature. The cations of ILs are alkylimidazolium, alkylprydinium, alkylammonium, etc., which consist of core parts with positive electric charges and alkyl-chains with hydrophobic interactions. On the other hand, halogen ions and many ionic groups, e.g., BF4–, PF6–, (CF3SO3)–, (CF3SO2)2N–, can be the anions of ILs. ILs have various useful properties as green solvents and electrochemical materials, e.g., low vapor-pressure, non-combustivity, high electric conductivity, etc. ILs have therefore been investigated mainly from the interests of application and industry. However, the basic physical properties of ILs, especially at low temperatures, have not been studied well. The most characteristic feature of ILs is the formation of domain structures as shown in Fig.1(b); ionic domains consisting of polar parts (imidazolium rings) of cations and anions and neutral domains of alkyl-chains of cations. The domain structure may play key roles for various interesting properties of ILs, e.g., stability of undercooled state, high fragility, non-Einstein-Stokes dynamics, etc. The purpose of this study is to clarify the structure and dynamics of the domains of imidazolium-based ILs.
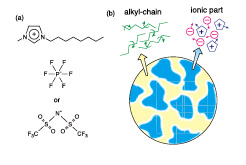
Fig. 1. Schematic drawings of (a) ionic structures of C8mimPF6 and C8mimTFSI, and (b) their domain strictures.
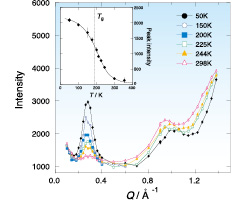
Fig. 2. Neutron diffraction data of C8mimPF6. The inset shows the temperature dependence of the intensity of the peak at 0.3 Å-1.
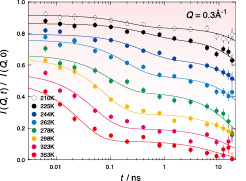
Fig. 3. NSE data of C8mimPF6 at Q = 0.3 Å-1.
We have performed neutron diffraction and spin echo experiments on 1-octyl-3-methylimidazolium hexafluorophosphate (C8mimPF6) and 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl) imide (C8mimTFSI), whose ionic structures are shown in Fig.1(a), using an NSE instrument at NCNR, NIST (USA). This instrument with using the wavelength of 6 Å can provide relaxation data in the Fourier time region of 6 ps to 17.5 ns. These two samples were chosen since clear domain structures and relatively fast domain motions are expected for ILs with longer alkyl-chain and larger anions. Both samples were fully deuterated to observe coherent scattering from the atoms forming the ions.
Figure 2 shows the neutron diffraction data of C8mimPF6. Two clear peaks appeared at 1.0 Å-1 and 0.3 Å-1. From the previous X-ray and neutron diffraction data and also computer simulation data, the former peak may be due to the correlation between the ions and the latter due to the correlation between the domains. On cooling, the ionic peak slightly shifted to the high-Q side, while the intensity of the domain peak drastically increased. It is of interest that the peak intensity still increases even below the glass transition temperature Tg as shown in the inset of Fig. 2. This result indicates that the atoms forming domains are still fluctuating even below Tg. Similar data were obtained also for C8mimTFSI.
Figure 3 shows the NSE data, intermediate scattering functions, of C8mimPF6 at Q = 0.3 Å-1. The data are fitted well to two exponential functions, corresponding to slower and faster relaxations, with two adjustable prefactors. It is noteworthy that, on cooling the sample, the fraction of the slower relaxation becomes larger while that of the faster relaxation smaller. Taking the diffraction data described above into consideration, the slower relaxation originates from the domains and the faster one from some sort of broken domains. This is the first experimental data demonstrating the relaxation of the domains. We are now planning the NSE experiment using neutrons with the wavelength 10 Å to extend the time region up to 50 ns. Then, clear relaxation data will be obtained at lower temperatures. The temperature dependence of the domain relaxation will give important information to clarify the mechanism of the domain formation in ILs.
